Dietary Supplement Gmp Haccp Fda

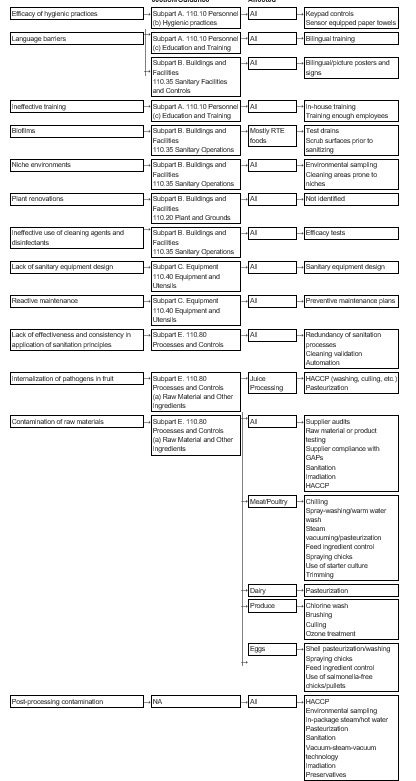
Haccp is a management system in which food safety is addressed through the analysis and control of biological chemical and physical hazards from raw material production procurement and handling.
Dietary supplement gmp haccp fda. In fiscal year 2019 fy19 dietary supplement manufacturing facilities issued an inspection report or so called form 483 for violations of cgmps current good manufacturing practices were most commonly cited for failing to establish specifications for. Guidance and regulatory information on food and dietary supplements. Fda is disappointed with continuing violations of basic manufacturing requirements applicable to the dietary supplement industry.
Small entity compliance guide december 2010 content current as of. Gmp dietary supplements training 21cfr111 gmp course. Includes guidance for industry as well as manufacturing processes food facility registration haccp retail food protection.
Food dietary supplement regulatory compliance training through online webinars seminars. After a relatively quiet 2017 2018 marked mostly by noticeable decline in fda inspections of dietary supplements for gmp good manufacturing practices compliance anecdotal evidence indicates the agency has returned to the field and that many of the issues that existed before continue to be gnawing problems for the industry today. The content of npa s dietary supplement gmp notes may relate to the final fda gmps or the npa gmp standards depending on the focus of the issue being addressed.
Current good manufacturing practice in manufacturing packaging labeling or holding operations for dietary supplements. This section includes training on fda gmp regulations food safety audit and inspection haccp iso 22000 fsma packaging labeling claims storage distribution requirements quality safety food marketing recalls crisis management more. Dietary supplements fda cgmp training 21cfr111 pd631846 fee.