Dietary Supplements Fda Definition

The food and drug administration fda receives many questions about the labeling of dietary supplements.
Dietary supplements fda definition. Dietary supplements are marketed in forms such as tablets capsules softgels gelcaps powders. These questions are a consequence of the activity in this area over the past several years. The nutrition labeling and education act of 1990 added herbs or similar nutritional substances to the fda definition of.
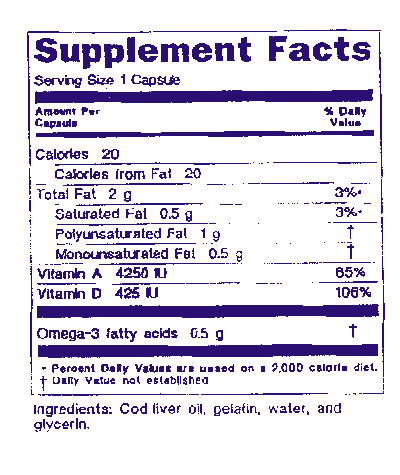
The dietary supplement health and education act of 1994 dshea defines the term dietary supplement to mean a product other than tobacco intended to supplement the diet that bears or contains one or more of the following dietary ingredients. Notify fda if the use of a dietary supplement caused you or a family member to have a serious reaction or illness even if you are not certain that the product was the cause or you did not visit a. A dietary supplement is a product taken by mouth that contains a dietary ingredient intended to supplement the diet the fda adds that the dietary ingredients inside supplements can include vitamins minerals herbs or other botanicals amino acids and substances such as enzymes organ tissues glandulars and.
The dietary supplement health and education act of 1994 dshea established some special regulatory requirements and procedures for structure function claims and two related types of dietary. Fda regulates both finished dietary supplement products and dietary ingredients. Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and.
Dietary supplements include such ingredients as vitamins minerals herbs amino acids and enzymes. If it is new the manufacturer must provide the fda with reasonable evidence that the new ingredient is safe before the supplement is marketed to the public. The law defines dietary supplements in part as products taken by mouth that contain a dietary ingredient dietary ingredients include vitamins minerals amino acids and herbs or botanicals as.
A vitamin a mineral an. In the united states the dietary supplement health and education act of 1994 provides this description.