Define Fda Dietary Supplement

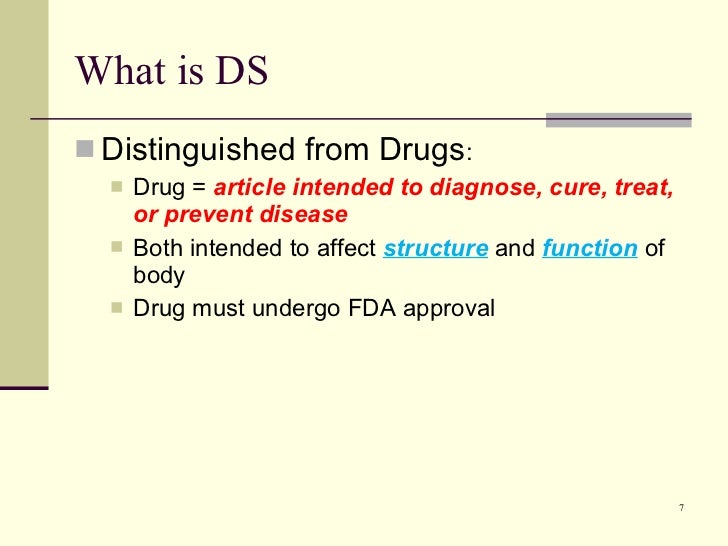
The fda used to consider dietary supplements to be composed only of essential nutrients such as vitamins minerals and proteins.
Define fda dietary supplement. Fda has asserted cbd is excluded from the definition of a dietary supplement because it was previously the subject of substantial clinical investigations instituted and made public. The nutrition labeling and education act of 1990 added herbs or similar nutritional substances to the fda definition of. The fda definition of dietary supplement is a tool of manipulation used by the banking mafia.
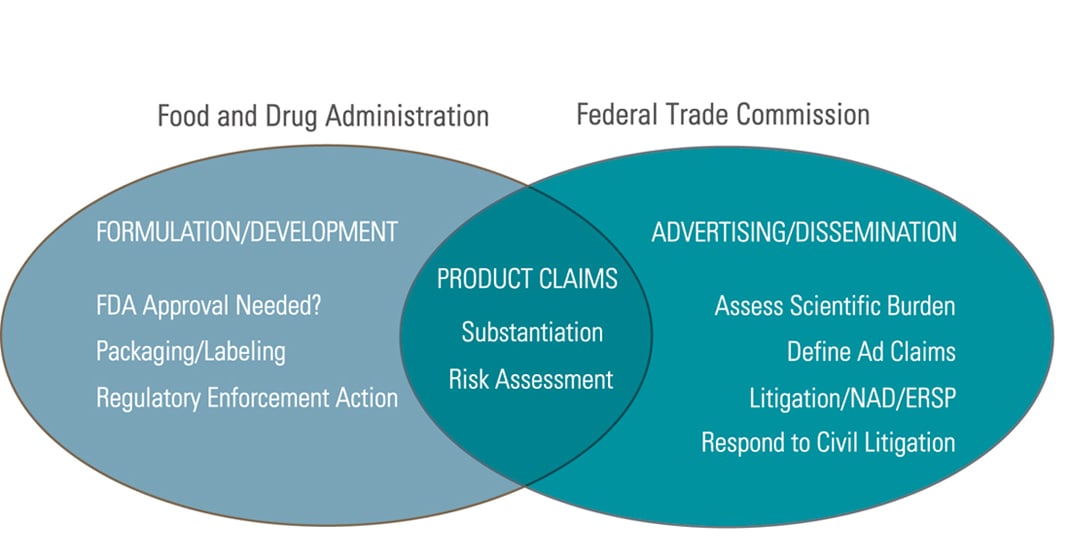
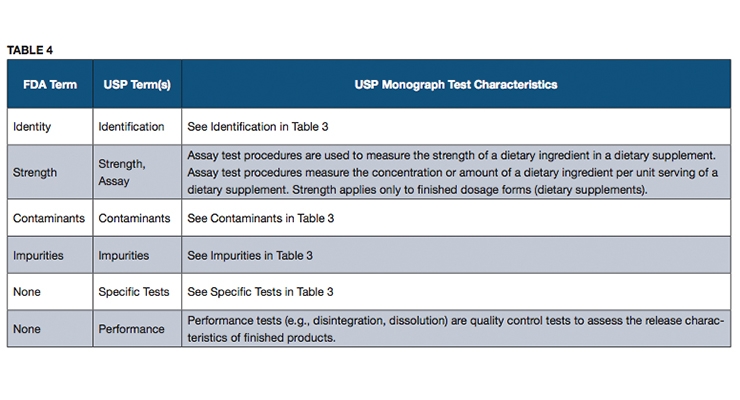
Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and. Fda regulates both finished dietary supplement products and dietary ingredients. The dietary supplement health and education act of 1994 the dshea amended the act in part by defining dietary supplements adding specific labeling requirements for dietary supplements and.
The dietary supplement health and education act of 1994 dshea established some special regulatory requirements and procedures for structure function claims and two related types of dietary. Gw pharmaceuticals plc which conducted the clinical investigations markets an fda approved cbd drug epidiolex for the treatment of seizures associated with two. Dietary supplement definition is a product taken orally that contains one or more ingredients such as vitamins or amino acids that are intended to supplement one s diet and are not considered food.
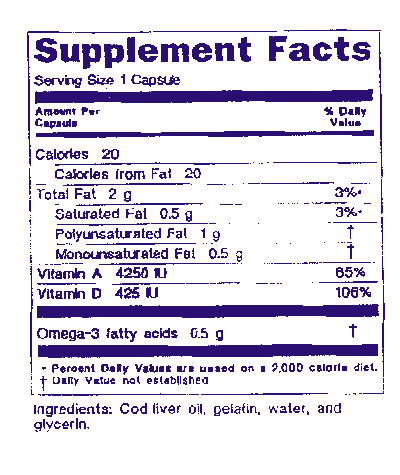
A dietary supplement is a manufactured product intended to supplement the diet when taken by mouth as a pill capsule tablet or liquid. The law defines dietary supplements in part as products taken by mouth that contain a dietary ingredient dietary ingredients include vitamins minerals amino acids and herbs or botanicals as. How to use dietary supplement in a sentence.