Us Pharmacopeia Dietary Supplement Verification Program

Over 100 different dietary supplement formulas have received the usp verified mark representing several different brands and retailers.
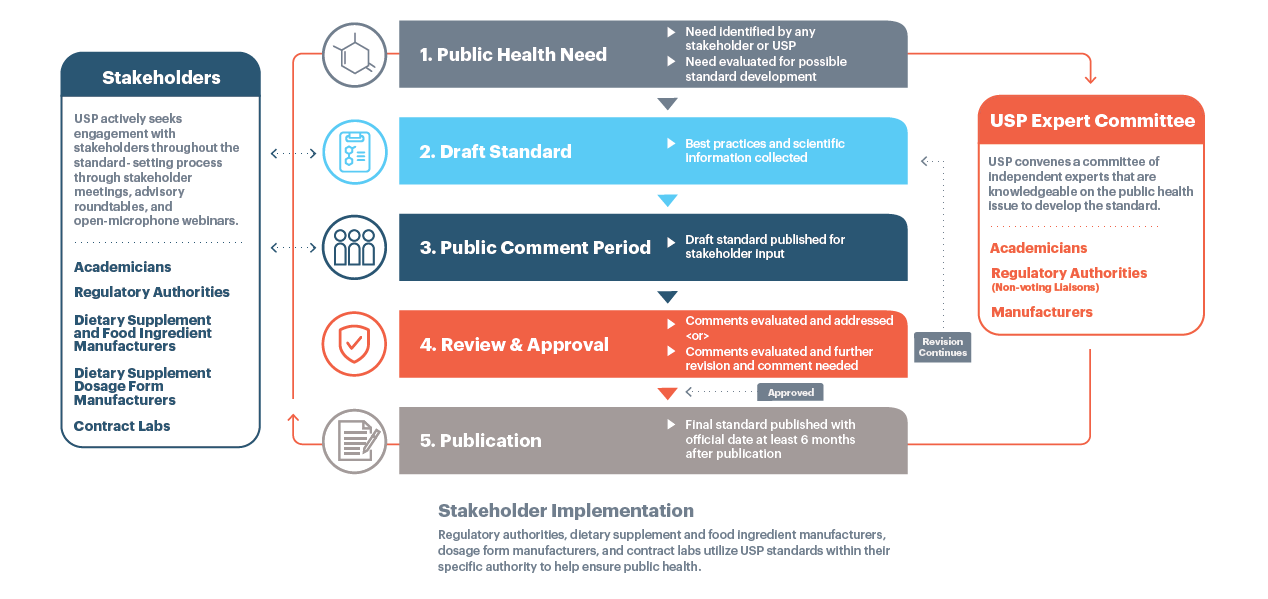
Us pharmacopeia dietary supplement verification program. The program serves as a means of supplier qualification potentially reducing audits from customers and reduces the risk of inconsistent and substandard ingredient quality. Review the most current list of dietary supplements verified by usp. Commonly used dietary supplement in the us 5 and were marketed in the us before 15 october 1994.
Usp prides itself in its dietary supplement verification program which consists of rigorous testing and auditing to evaluate whether submitted products meet the science based quality standards of quality purity potency performance and consistency. Thus these products do not require fda premarket table ii. Usp is committed during these extraordinary times to provide support to manufacturers to help ensure the quality of dietary supplements by providing auditing and testing services to verify the quality of dietary supplements through the usp verification program.
Usp nf is a combination of two compendia the united states pharmacopeia usp and the national formulary nf. Several brands voluntarily participate in the usp dietary supplement verification program. Dietary ingredient verification program the program can differentiate ingredients and help a company maintain its sales edge in a competitive global market.
The dietary supplements quality collaborative dsqc comprised of 27 organizations committed to safeguarding public health is hosting a webinar on advancing the quality and safety of dietary supplements to discuss modernizing and strengthening the current regulatory framework. Monographs for drug substances dosage forms and compounded preparations are featured in the usp. Dietary supplements verification program when it comes to dietary supplements many consumers and healthcare practitioners look for independent oversight from a third party preferably a fully independent outsider not associated with the manufacturer and with the necessary expertise to assess quality.
Join us on september 17 at 2 pm et. Monographs for dietary supplements and ingredients appear in a separate section of the usp. The mark can be used on product labeling packaging and promotional materials to help distinguish usp verified products in the marketplace and aid consumers in their decision making process.