Dietary Supplements Regulation In Canada

Supplement regulation varies by country and even within countries so it d be quite an extensive project to write an article covering all jurisdictions on the planet.
Dietary supplements regulation in canada. Nutritional supplements to which milk partially skim milk or skim milk is to be added must carry a statement that the nutrient content of the food has been determined taking into consideration the milk partially skim milk or skim milk that is to be added according to the directions for use b 24 202 b fdr. As a result i will specifically discuss the federal regulation of dietary supplements in the united states for this article. Differences with food and drug regulation in these countries will be noted as well.
Dietary supplements are commonly used by canadian armed forces caf personnel to enhance performance. How dietary supplements are regulated in canada sovereign silver and argentyn 23 set the industry benchmark in terms of safety and efficacy and were the first colloidal silver products approved for ingestion by any federal agency in the world. Links to related regulations legislation and guidelines including 53 recommendations of the standing committee on health.
It also provides access to proposed and recently adopted legislative and regulatory initiatives related to food and nutrition at health canada. In canada dietary supplements are called natural health products nhps which is a subcategory under drugs regulated by natural health products regulations nhpr. These products promise muscle gain weight loss increased endurance increased muscle power reduced fatigue improved appearance accelerated workout recovery and slowed aging.
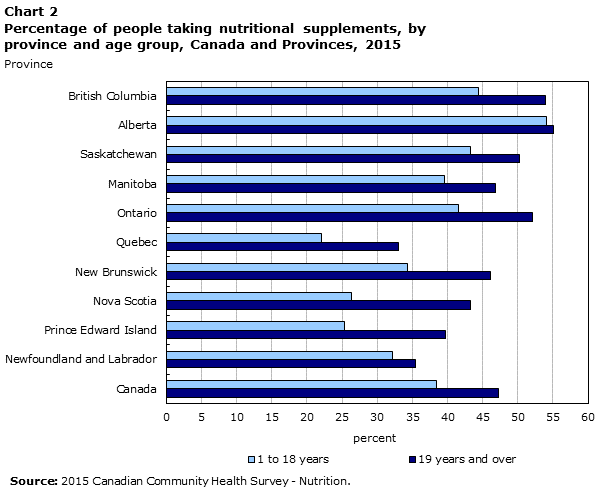
26 a survey in 2010 by ipsos reid shows that 73 canadians regularly take nhps including vitamins minerals herbal products and homeopathic medicines. Dietary supplement legislative history.