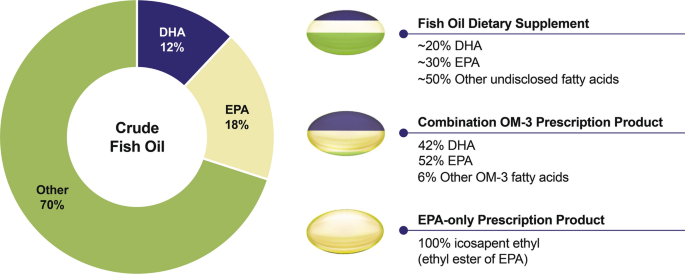
Dietary Supplements Have Regulations That Are Strict As Compared To Drugs

The dietary supplement health and education act accomplished which of the following.
Dietary supplements have regulations that are strict as compared to drugs. Supplements aren t regulated by the fda. Dietary supplements are considered safe until proven unsafe. In 1994 the dietary supplement health and education act dshea defined dietary supplements as a category of food which put them under different regulations than drugs.
Dietary supplements have regulations that are strict as compared to drugs. Women of childbearing age should consume daily from fortified foods or supplements. Supplements of which of the following nutrients may be needed by older adults.
Guidance and regulatory information on food and dietary supplements. Dietary supplements have regulations that are strict as compared to drugs. Dietary supplements have regulations that are strict as compared to drugs.
The general rule is drugs are considered unsafe until they re proven safe. Under the dietary supplement health and education act dshea the fda treats supplements like food and the dshea defines supplements as products taken orally for supplementing the diet supplements. A less b equally c more.
The dietary supplement health and education act of 1994 dshea. Structure function claims have historically appeared on the labels of conventional foods and dietary supplements as well as drugs. The dietary supplement health and education act accomplished which of the following.
A defined the term dietary supplement and established safety standards for manufacturing practices.