Fda Dietary Supplement Structure Function Claims

What laws and regulations govern structure function claims.
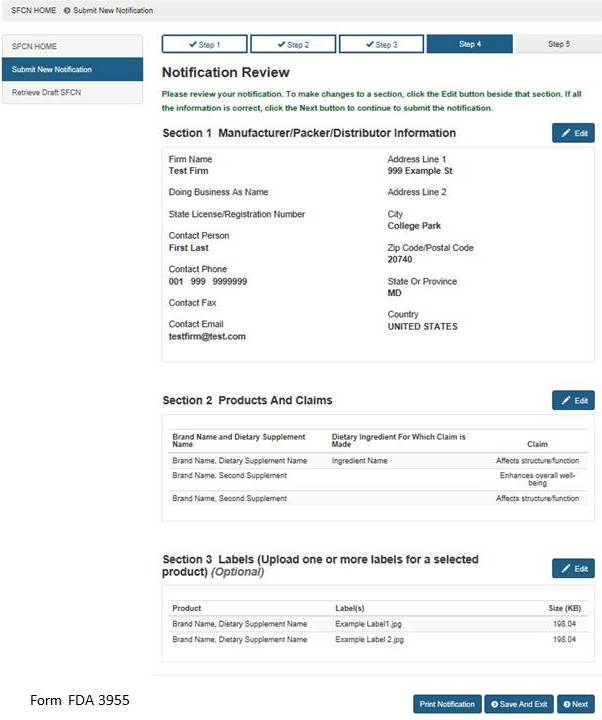
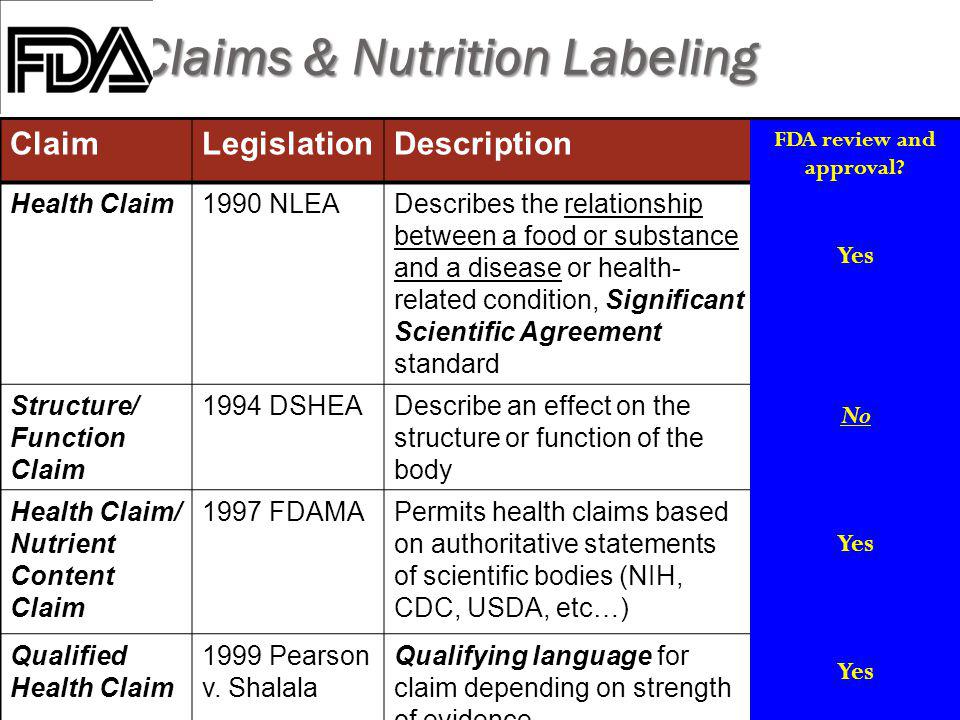
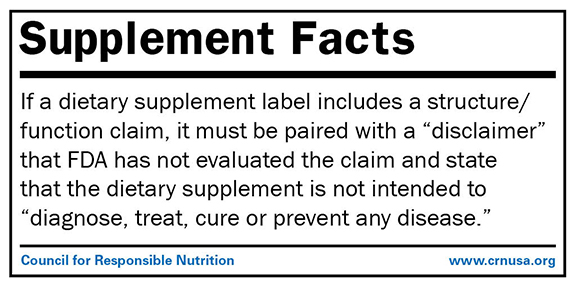
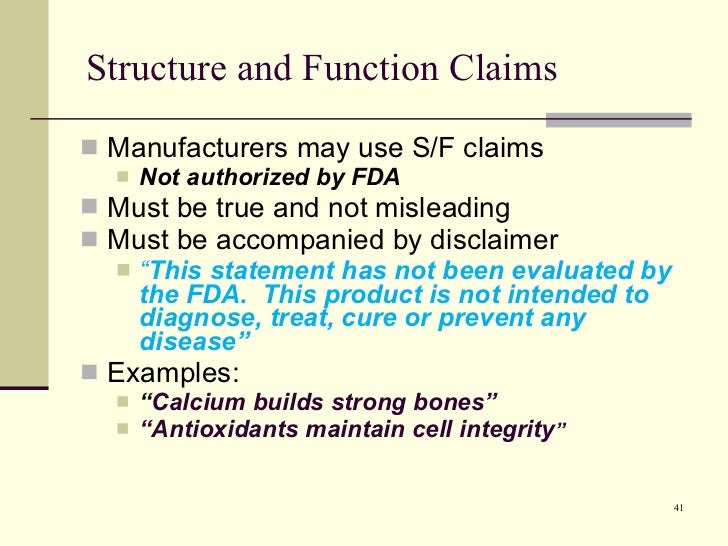
Fda dietary supplement structure function claims. Finally the notification must be submitted to fda no later than 30 days after the first marketing of the dietary supplement product. The dietary supplement health and education act of 1994 dshea established some special regulatory requirements and procedures for structure function claims and two related types of dietary. The fda permits supplements to so called structure function claims which are claims that describe the role of a nutrient or dietary supplement intended to affect the structure or function in humans or characterize the documented mechanism by which the nutrient acts to maintain such structure or function but do not claim to.
What is a structure function claim. Content current as of. For more information see fda modifies internal processes for new dietary ingredient notification and structure function claim notification submissions.
This section of the law states that a dietary supplement may bear structure function claims and provides their requirements. 2 characterize the action by which a nutrient or dietary ingredient maintains such structure or function fiber helps.