Dietary Supplement Finished Product Specification

Smart specifications are developed maintained and implemented in an efficient manner.
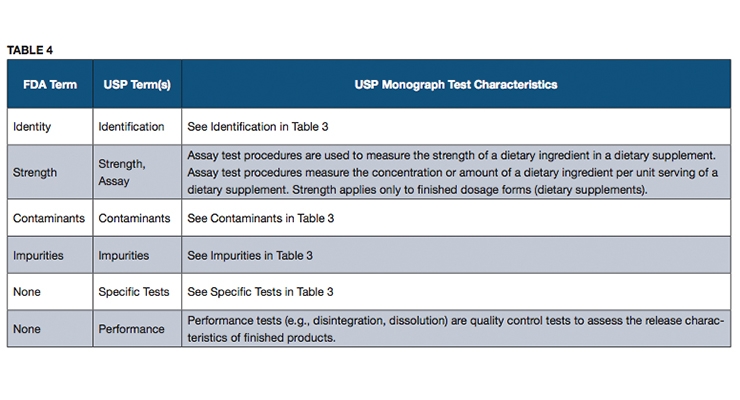
Dietary supplement finished product specification. 39 inspections 12 30 percent. 111 70 c labels and packaging final sec. A part of the 2007 fda gmps 21 cfr 111 for manufacturing dietary supplements requires having specifications.
Finally finished product specifications fp establish the identity purity strength composition and limits of contaminates for each finished batch of dietary supplement. Final report or interim report and corrective action review issued after audit. 111 70 d the finished batch of dietary supplement final sec.
111 gmp audit conducted every two years at the facility s that manufacturer the finished dietary supplement or natural product. Does the new rule require finished product testing. Finished product specifications detail the dietary supplement finished batch testing requirements.
111 70 b in process production final sec. In short the finish product specification details testing requirements for a finished batch. Every claimed dietary ingredient listed on the supplement facts panel sfp must be addressed on the finished product specification and each must have an appropriate minimum test acceptance criterion that meets each product specification for.
Establish specifications for components final sec. 111 70 f and the packaging and labeling for the finished packaged and labeled dietary. 111 70 e product that you receive from a supplier for packaging and labeling final sec.
All dietary ingredients listed on the sfp must be identified on the fp. Raw materials in process finished products and packaged products. Yes the final fda gmps requires you to verify that every finished batch of dietary supplement meets each product specification for identity purity strength composition and for limits on contamination that may adulterate your product.