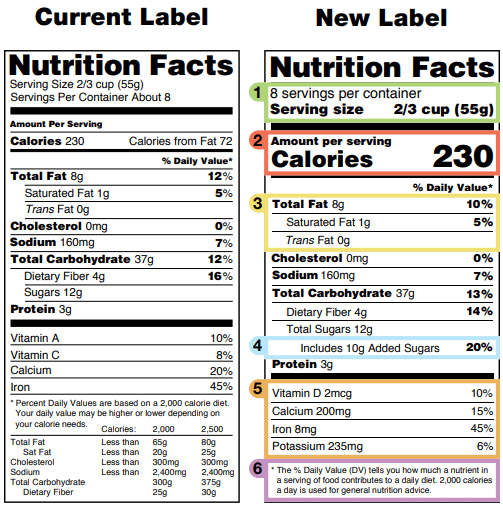
Fda Dietary Supplement Labeling Examples

Indeed ensuring that all information concerning that particular dietary supplement is in compliance with fda labeling requirements may be difficult and often very onerous.
Fda dietary supplement labeling examples. The food and drug administration fda receives many questions about the labeling of dietary supplements. Food and drug administration today issued draft guidance to advise firms that manufacture market or distribute dietary supplements that contain live microbial ingredients of the. If there are any others i would like to know about them.
Industry stakeholders found points of. For example fda reviews substantiation for claims as resources permit. There are limitations to fda oversight of claims in dietary supplement labeling.
Listed on the product s label. The american medical association has issued a new policy on dietary supplements that calls for more enforcement from fda labeling changes and other measures. A full scale contract manufacturer of nutraceuticals and dietary supplements.
Fda s interim procedures for qualified health claims in the labeling of conventional human food and human dietary supplements describes the agency s process for considering petitions for the use. As far as the fda goes i have always found it hard to find guidance on sample products i would follow the labeling guidance in the fda food labeling guide found here aside from the exception for nft info on donated products i have not found any other exceptions. Labeling of processing aids.
For example for a product to qualify for a high in antioxidant vitamin c claim it must contain 20 percent or more of the rdi for. Examples of process aids used in dietary supplements. These questions are a consequence of the activity in this area over the past several years.
In line with fda s requirements process aids are not required to be listed on the supplement label even though some trace amounts of the material may remain in the product.