Fda Dietary Supplement Definition

An ingredient includes but is not necessarily limited to a dietary ingredient as defined in section 201 ff of the act.
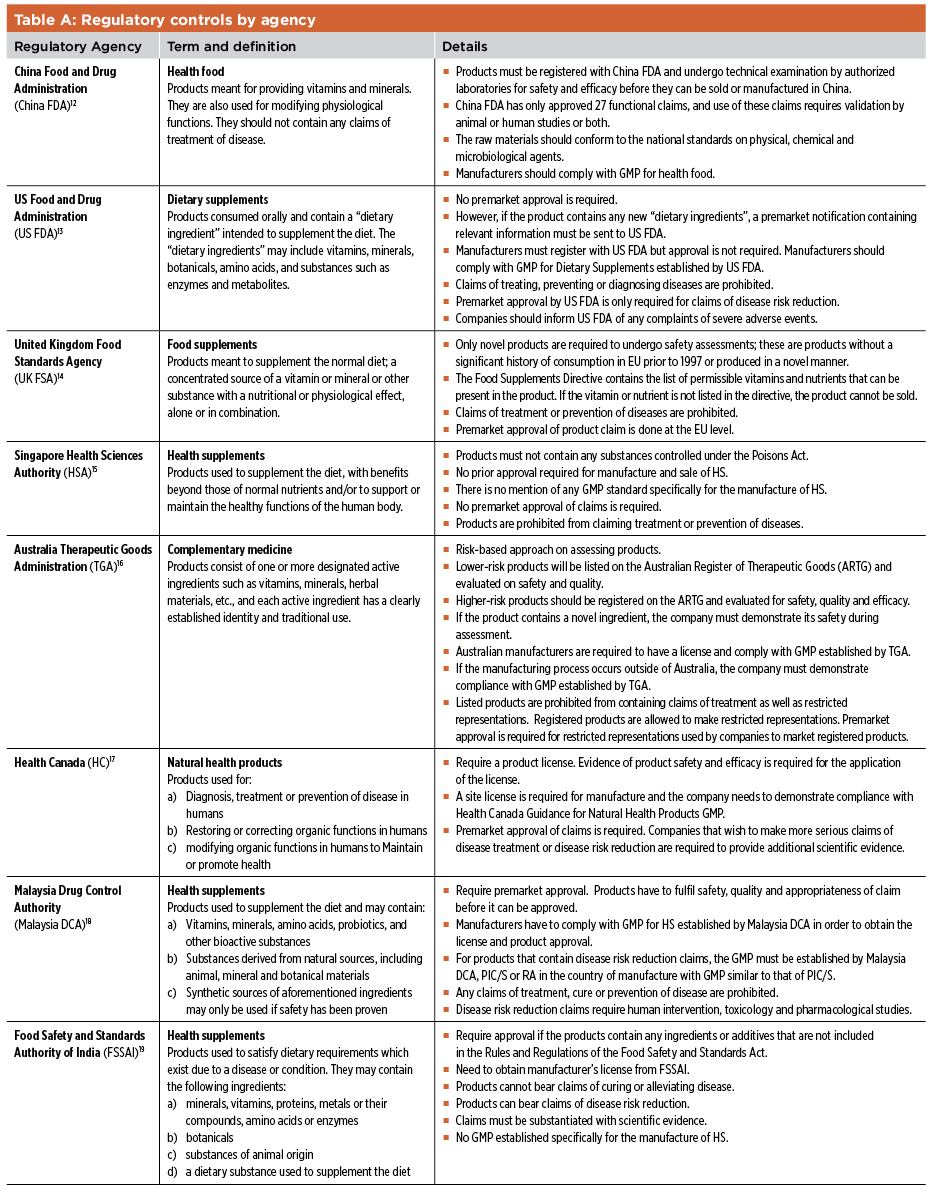
Fda dietary supplement definition. Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and. A dietary supplement is a product taken by mouth that is intended to supplement the diet and that contains a dietary ingredient. The law defines dietary supplements in part as products taken by mouth that contain a dietary ingredient dietary ingredients include vitamins minerals amino acids and herbs or botanicals as.
Ingredient means any substance that is used in the manufacture of a dietary supplement and that is intended to be present in the finished batch of the dietary supplement. Fda announced the nutrition and supplement facts label final rule which included a definition of dietary fiber and identified seven isolated or synthetic non digestible. The dv must be declared for all dietary ingredients for which fda has established daily values except that 1 the percent for protein may be omitted and 2 on the labels of dietary supplements.
The dshea gives the fda permission to stop a company from making a dietary supplement but only when the fda proves that the product poses a significant risk to the health of americans. This is the reverse of the way prescription and non prescription drugs are handled. The dietary ingredients in these products can include vitamins minerals herbs and other botanicals amino acids other dietary substances intended to supplement the diet and concentrates.
Fda regulates both finished dietary supplement products and dietary ingredients. Dietary supplement definition is a product taken orally that contains one or more ingredients such as vitamins or amino acids that are intended to supplement one s diet and are not considered food. The fda definition of dietary supplement is a tool of manipulation used by the banking mafia.
This means they are found unsafe only after they cause harm. The fda used to consider dietary supplements to be composed only of essential nutrients such as vitamins minerals and proteins.