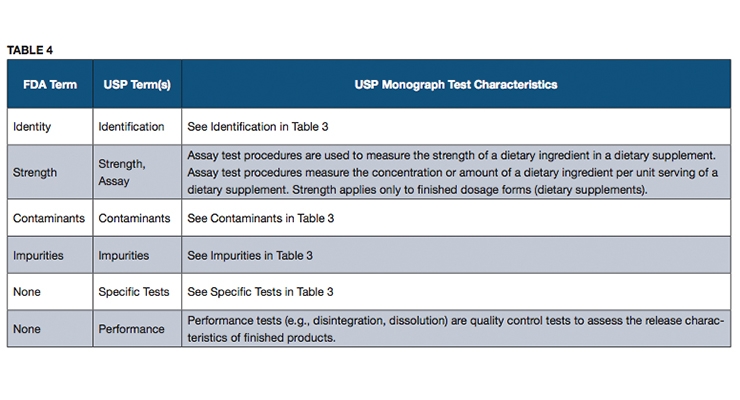
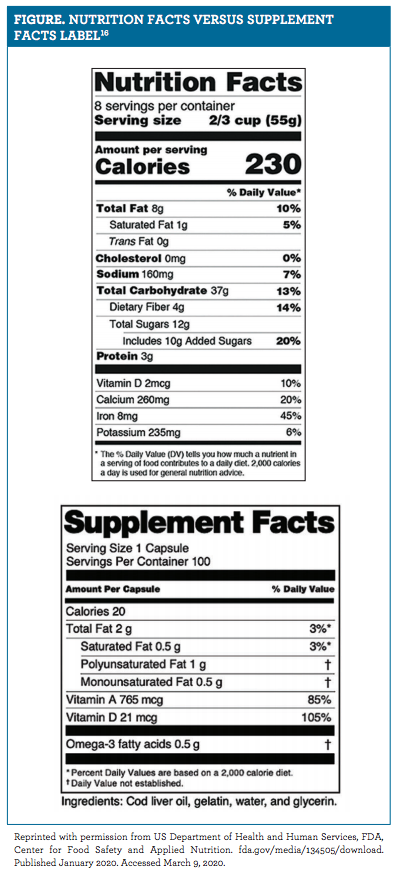
Fda Definition Of Adulterated Dietary Supplement

The adulteration of dietary supplements has been reported fairly frequently yet most consumers and health care professionals are unfamiliar with the problem.
Fda definition of adulterated dietary supplement. Adulteration of dietary supplements typically involves 1 of 2 patterns. Oversight of the fda s foreign drug manufacturing inspection process. Office of dietary supplement programs hfs 810 food and drug administration 5001 campus dr college park md 20740.
5 definition of dietary supplement product other than tobacco that is intended to supplement the diet product that is intended for ingestion contains one or more dietary ingredients www fda gov dietary substance for use by man to. Under existing law including the dietary supplement health and education act passed by congress in 1994 the fda can take action to remove products from the market but the agency must first. Npa submitted comments for the record to the senate finance committee s hearing covid 19 and beyond.
Economic adulteration in which a less expensive ingredient is used in place of a more expensive ingredient listed on the label or pharmaceutical adulteration in which an active drug is included in a purportedly botanical supplement for example sildenafil in a. The law defines dietary supplements in part as products taken by mouth that contain a dietary ingredient dietary ingredients include vitamins minerals amino acids and herbs or botanicals as. Npa has asked the fda issue an import alert for dietary supplements or ingredients that fail to comply with new dietary ingredient regulations.
Food and drug administration fda to issue import alerts for nutritional supplements that fail to comply with new dietary ingredient ndi regulations. Such ingredients have been found to be unsafe lack evidence of safety don t meet the definition. The food drug administration fda takes action against dietary supplement companies for selling products identified as being adulterated or misbranded the former is especially important because it involves the presence of ingredients not permitted for use in dietary supplements.
The natural products association npa urged the u s.