Dietary Supplements Fda Product Code
The law defines dietary supplements in part as products taken by mouth that contain a dietary ingredient dietary ingredients include vitamins minerals amino acids and herbs or botanicals as.
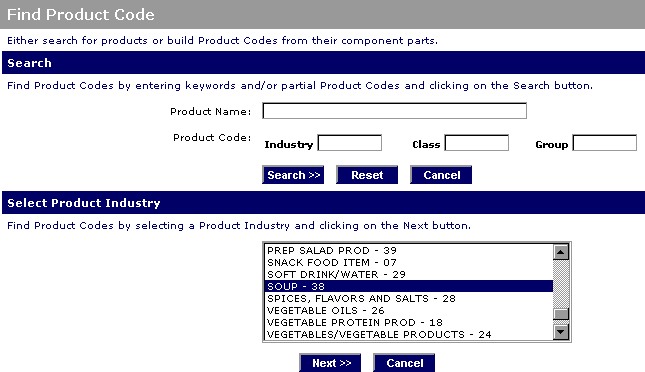
Dietary supplements fda product code. Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and drug products. A dietary supplement is a product taken by mouth that contains a dietary ingredient intended to supplement the diet the fda adds that the dietary ingredients inside supplements can include vitamins minerals herbs or other botanicals amino acids and substances such as enzymes organ tissues glandulars and. By building upon the code portions you select the application will provide valid choices for each of the five components of the product code industry class subclass.
Guidance and regulatory information on food and dietary supplements. The application returns the primary. Our dietary supplement import data solutions meet your actual import requirements in quality volume seasonality and geography.
Includes guidance for industry as well as manufacturing processes food facility registration haccp retail food protection. Indeed ensuring that all information concerning that particular dietary supplement is in compliance with fda labeling requirements may be difficult and often very onerous. This element is two numbers from 02 to 98 an industry code determines the broadest area into which a product falls.
A 1 no later than 30 days after the first marketing of a dietary supplement that bears one of the statements listed in section 403 r 6 or the federal food drug and cosmetic act the manufacturer packer or distributor of the dietary supplement shall notify the office of nutritional products labeling and dietary supplements hfs 810 center for food safety and applied nutrition food. Alongside we help you get detailed information on the vital import fields that encompass hs codes product description duty quantity price etc. If you have a product code and want to know if it is still a valid code or if you are not sure what product it represents you can enter the code in the appropriate fields.
If the product code is valid the name of the product will appear on the next screen. Under the dietary supplement health and education act of 1994. Fda labeling is one of the most important regulatory requirements for dietary supplements in that it provides the consumer the necessary information on the product.