Dietary Supplement Finished Product Specification Template

111 70 c labels and packaging final sec.
Dietary supplement finished product specification template. 111 70 d the finished batch of dietary supplement final sec. 111 70 e product that you receive from a supplier for packaging and labeling final sec. In week 33 of the haccp mentor food safety haccp challenge finished product specifications are in the spotlight.
Product specification template author. Labelling not required in accordance with fsanz food standards code standard 1 5 2. Cereals containing gluten wheat rye barley oats.
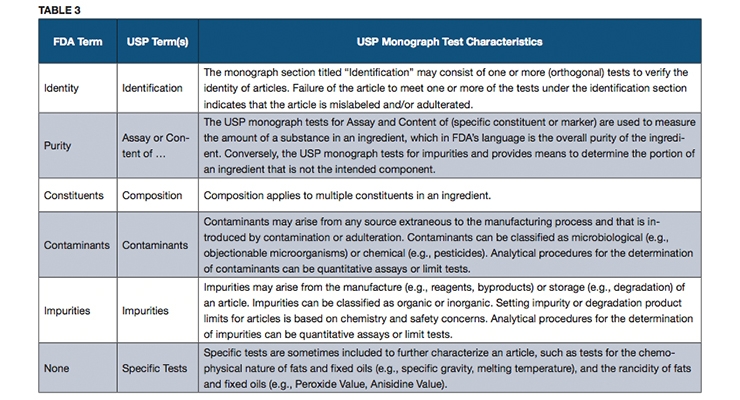
111 70 b in process production final sec. All dietary ingredients listed on the sfp must be identified on the fp. Finally finished product specifications fp establish the identity purity strength composition and limits of contaminates for each finished batch of dietary supplement.
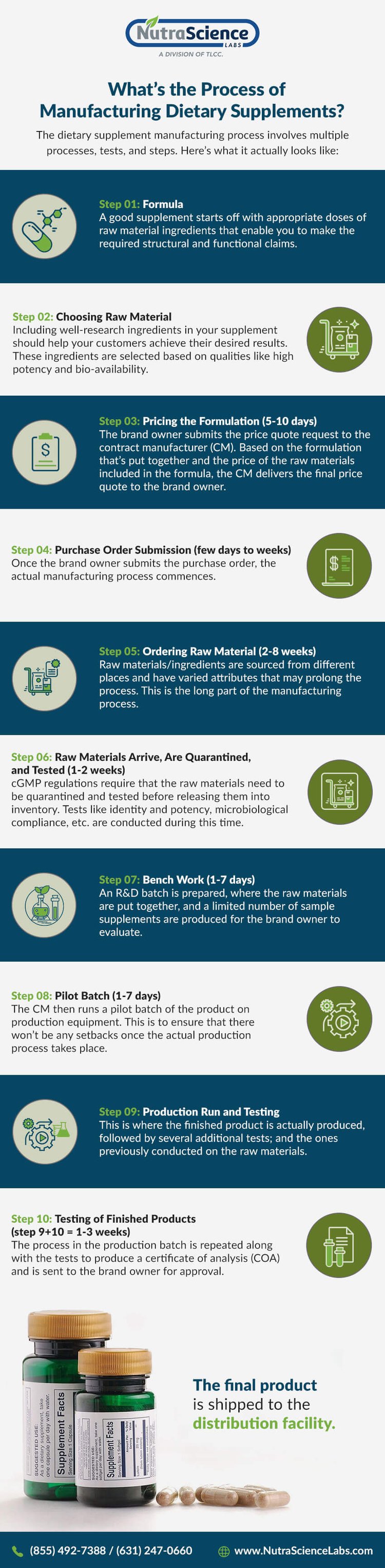
A finished product specification is the information sheet that documents all of the attributes and information regarding the finished product that you produce in your food business. Specify total protein in. The manufacture of a dietary supplement product.
Process controls qc checks parameter method by whom frequency target comments. It provides recommendations to comply with the regulatory requirements of component supplier qualification and may be utilized by finished product manufacturers or other users of dietary supplement components to build the foundation for a supplier qualification program. Genetic modification spl does not used genetically modified materials in its products.
This guideline is applicable to all dietary supplement components defined as both dietary ingredients and other ingredients used in the manufacture of a dietary supplement product. This guideline focuses on compliance with us regulations but has international. Establish specifications for components final sec.