Usp Dietary Supplement Chapter

The use of official usp reference standards is encouraged for verification purposes.
Usp dietary supplement chapter. It is a growth medium for a variety of microorganisms that present a threat to product quality safety preservation and stability. Usp dsc also includes gmp associated general chapters for. Vitamin mineral dosage forms includes articles prepared with vitamins minerals or combinations of these dietary ingredients e g usp dietary.
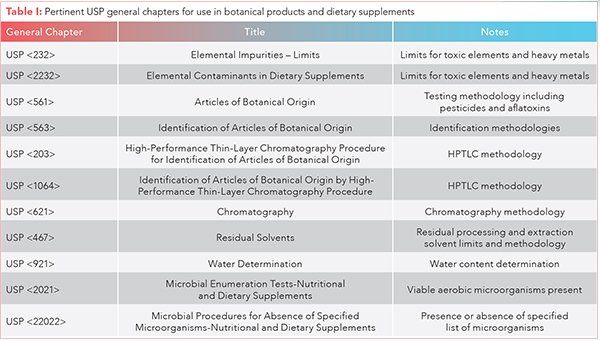
This general chapter is not intended to set limits for dietary ingredients. Limits for dietary ingredients are set in the corresponding individual monographs. 180 excipient monographs relevant to ds manufacturers.
The principles set forth in this chapter contain recommended minimum current good manufacturing practices for the methods to be used in and the facilities and controls to be used for the manufacture of a dietary supplement to assure that such a product meets the requirements of safety and has the identity and strength and meets the quality and purity characteristics that it is represented. 220 food chemicals codex fcc monographs and 17. Alternative methods may be substituted for the tests provided that they.
This chapter provides tests for the estimation of the number of viable aerobic microorganisms present in nutritional supplements of all kinds from raw materials to the finished forms. 2021 microbial enumeration tests nutritional and dietary supplements. For the purposes of this chapter dietary supplement dosage forms have been divided into three categories.
The usp dietary supplements compendium dsc is one stop shop for all your ds quality needs including the necessary analytical tools such as monographs general chapters and chemical reference standards to conduct the necessary identity testing strength purity and performance. Vitamin mineral dosage forms botanical dosage forms and dietary supplements other than vitamin mineral and botanical dosage forms. This chapter describes the testing of nutritional and dietary articles for specified microorganisms which are specified in the individual monographs or whose absence is recommended by the guidance under microbiological attributes of nonsterile nutritional and dietary supplements 2023 when objectionable microorganisms are not specified in the individual monograph it is the manufacturers.
Water as one of the major components in nutritional and dietary supplement manufacturing processes water deserves a special consideration in the microbiological control of these articles. The objective of this general chapter 2232 is to limit the amounts of elemental contaminants in finished dietary supplement dosage forms labeled as conforming to usp or nf standards. 28 5 page 1548 phone number.