Dietary Supplement Adverse Event Reporting Guidance

Questions answers regarding adverse event reporting and recordkeeping for dietary supplements as required by the dietary supplement nonprescription drug consumer protection act issued in october 2007 is a helpful guide for compliance with saer requirements.
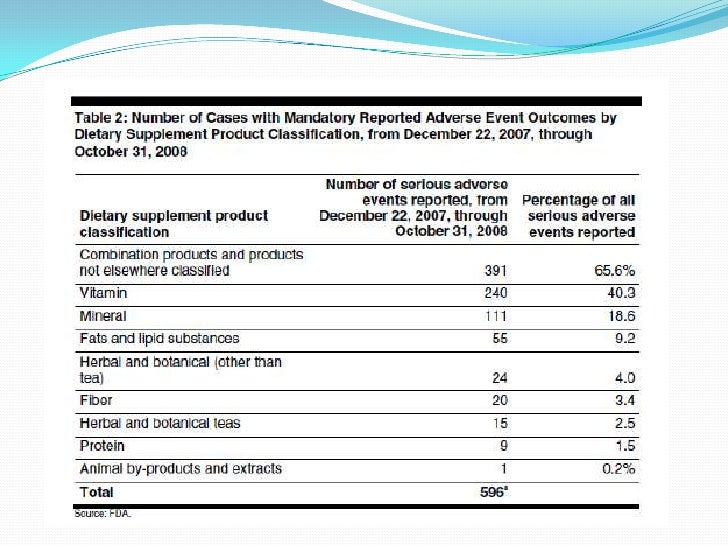
Dietary supplement adverse event reporting guidance. This guidance provides recommendations to industry regarding postmarketing adverse event reporting for drugs biologics medical devices and dietary supplements during a pandemic. Department of health and human services. This guidance document provides guidance to the dietary supplement industry for complying with the adverse event reporting and recordkeeping requirements prescribed for dietary supplement.
Electronic submission is voluntary. A manufacturer packer or. Guidance for industry postmarketing adverse event reporting for medical products and dietary supplements during a pandemic.