Fda Dietary Supplement Labeling Guide Pdf
These questions are a consequence of the activity in this area over the past several years.
Fda dietary supplement labeling guide pdf. Therefore the draft guidance provides no useful guidance to dietary supplement. Examples are an excellent way to provide guidance to industry but fda fails to include any examples here for dietary supplement products carrying supplement facts labels. The federal trade commission ftc and the food and drug administration fda work together under a long standing liaison agreement governing the division of responsibilities between the two agencies.
A dietary supplement labeling guide chapter iv. The food and drug administration fda receives many questions about the labeling of dietary supplements. Fda nutrition labeling manual a guide for developing and using data bases march 1998 label claims letter regarding point of purchase food labeling october 2009.
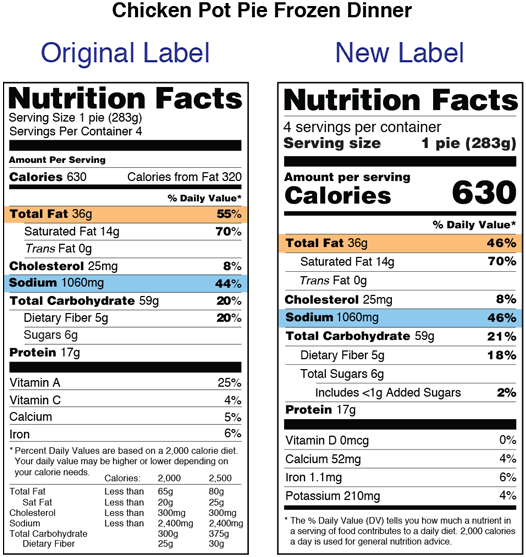
Five statements are required. Nutrition labeling for raw produce fruits and vegetables and. Almost two decades later on may 27 2016 fda published a final rule revising the nutrition labeling requirements for foods and dietary supplements.
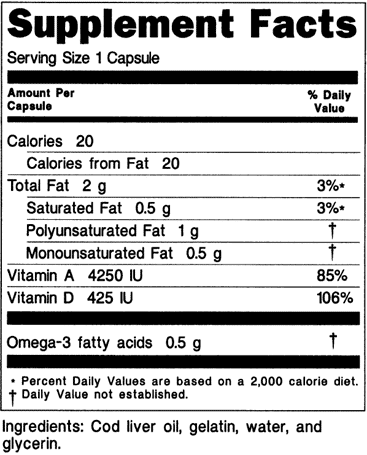
New food labeling draft guidance fails to provide examples for dietary supplements. The initial nutrition labeling requirements for dietary supplements were promulgated by the food and drug administration fda in 1999. 1 the statement of identity name of the dietary supplement 2 the net quantity of contents statement amount of the dietary supplement 3 the nutrition labeling.
Labeling and dietary supplements hfs 800 food and drug administration 5100 paint branch parkway college park. Food labeling is required for most prepared foods such as breads cereals canned and frozen foods snacks desserts drinks etc.