Dshea Dietary Supplement Label

Congress established the dietary supplement health and education act dshea in 1994 to create a regulatory framework to address the safety and labeling of dietary supplements.
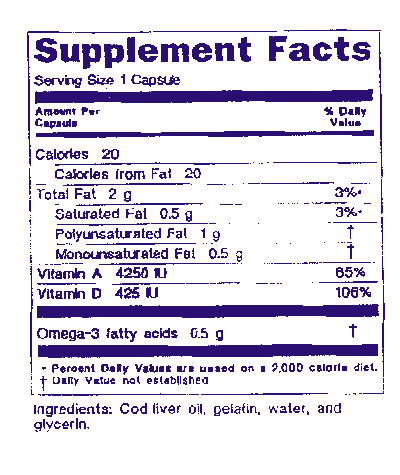
Dshea dietary supplement label. A statement of identity that contains the words dietary supplement. Dietary supplement ingredient labeling and nutrition information labeling. 1 the statement of identity name of the dietary supplement 2 the net quantity of contents statement amount of the dietary supplement 3 the nutrition labeling 4 the ingredient list and 5 the name and place of business of the manufacturer packer or distributor.
A vitamin a mineral an herb. More recent regulations require manufacturers to observe good manufacturing practices gmps established for this industry including ingredient testing. The dietary supplement health and education act of 1994 the dshea amended the act in part by defining dietary supplements adding specific labeling requirements for dietary supplements and.
To maintain the product s status as a dietary supplement the label and labeling must be consistent with the provisions in the dietary supplement health and education act dshea of 1994 labeling. Percentages are to only be listed for ingredients that have an established reference daily intake or daily recommended value. Dietary supplement labels.
S if 1 it is a dietary supplement. Section 403 21 u s c. An herb or other botanical.
The food and drugs administration fda in the u s. The dietary supplement health and education act of 1994 dshea defined a dietary supplement and permitted the addition of dietary ingredients if they meet the act s requirements 1 a dietary ingredient is a vitamin. Manufacturers of dietary supplements must ensure that their products are safe and accurately labeled as per fda supplement regulation.
Dshea and other federal regulations require the following information to appear on dietary supplement labels. Under dshea labeling requirements all supplements must identify the product as a dietary supplement and include the name and quantity of each ingredient and the identity of the part of the plant used. And 2 a the label or labeling of the supplement fails to list.