Dietary Supplement Regulation Canada

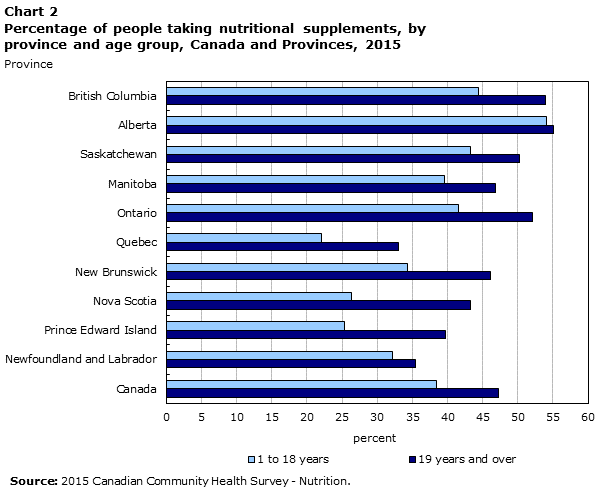
26 a survey in 2010 by ipsos reid shows that 73 canadians regularly take nhps including vitamins minerals herbal products and homeopathic medicines.
Dietary supplement regulation canada. During the development of the regulations health canada consulted with industry assessed business impact and revised the regulations to address industry concerns. More recent regulations require manufacturers to observe good manufacturing practices gmps established for this industry including ingredient testing. Health canada regulation of natural health products their licensing manufacturing clinical trials labelling and adverse reaction reporting.
Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and drug products. In canada dietary supplements are called natural health products nhps which is a subcategory under drugs regulated by natural health products regulations nhpr. Who will benefit executives managers within dietary supplement or natural product companies.
Dietary supplements are considered to be food products under the dietary supplements health education act so claims may not be made about the use of a dietary supplement to. Under the dietary supplement health and education act of 1994. Both general foods and dietary supplements could be identified by fda as misbranded when they fail to meet applicable label claims or labeling requirements provided in the 21 cfr 101 regulation.
An update on current events within the supplement industry and the potential impacts to manufacturers and distributors in the us eu and canada will also be presented and discussed. As a result i will specifically discuss the federal regulation of dietary supplements in the united states for this article. The canadian natural health products regulations require individuals to obtain a product license before they can sell a natural health product in canada.
Supplement regulation varies by country and even within countries so it d be quite an extensive project to write an article covering all jurisdictions on the planet. To obtain a license individuals must submit an application to the nnhpd which must include sufficient data to assess the safety quality and efficacy of the product. Links to related regulations legislation and guidelines including 53 recommendations of the standing committee on health.
Congress established the dietary supplement health and education act dshea in 1994 to create a regulatory framework to address the safety and labeling of dietary supplements.