Dietary Supplement Labeling Guide Pdf

Given the dramatic increase in the volume and variety of dietary supplement advertising in recent years ftc staff is issuing this guide to clarify how long standing ftc policies and enforcement practices relate to dietary supplement advertising.
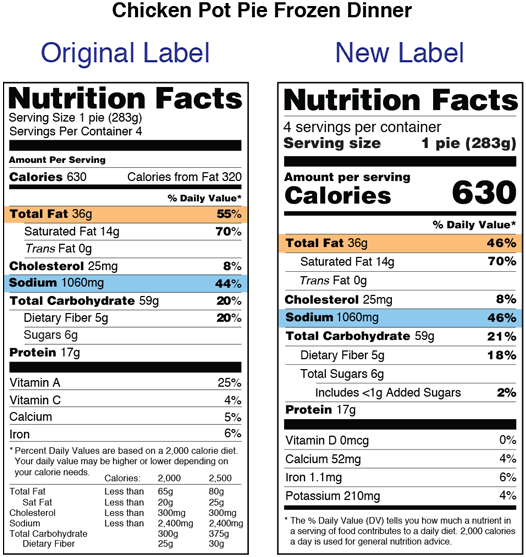
Dietary supplement labeling guide pdf. The initial nutrition labeling requirements for dietary supplements were promulgated by the food and drug administration fda in 1999. You should make a label that represents your brand and creativity at the same time you. Federal government websites often end in gov or mil.
Five statements are required. A food labeling guide office of nutrition labeling and dietary supplements hfs 800 center for food safety and applied nutrition food and drug administration 5100 paint branch parkway college park md 20740 tel 301 436 2375 updated phone. Dietary supplements š must be truthful not misleading and substantiated.
Food labeling guide september 1994. 1 the statement of identity name of the dietary supplement 2 the net quantity of contents statement amount of the dietary supplement 3 the nutrition labeling. A dietary supplement labeling guide chapter iv.
An easy and convenient way to make label is to generate some ideas first. Almost two decades later on may 27 2016 fda published a final rule revising the nutrition labeling requirements for foods and dietary supplements. In april 2005 we issued a guidance for industry entitled a dietary supplement labeling guide the guidance covers the most frequently raised questions about the labeling of dietary supplements using a question and answer format and is intended to help ensure that the dietary supplements sold in the united states are properly labeled.
The gov means it s official. Labeling and dietary supplements center for food safety and applied nutrition food and drug administration 5100 paint branch parkway college park md 20740 3835 telephone. Revised october 2009 guidance for industry.