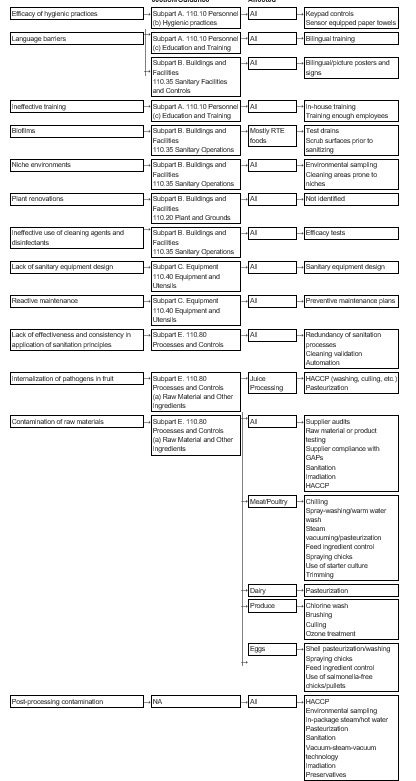
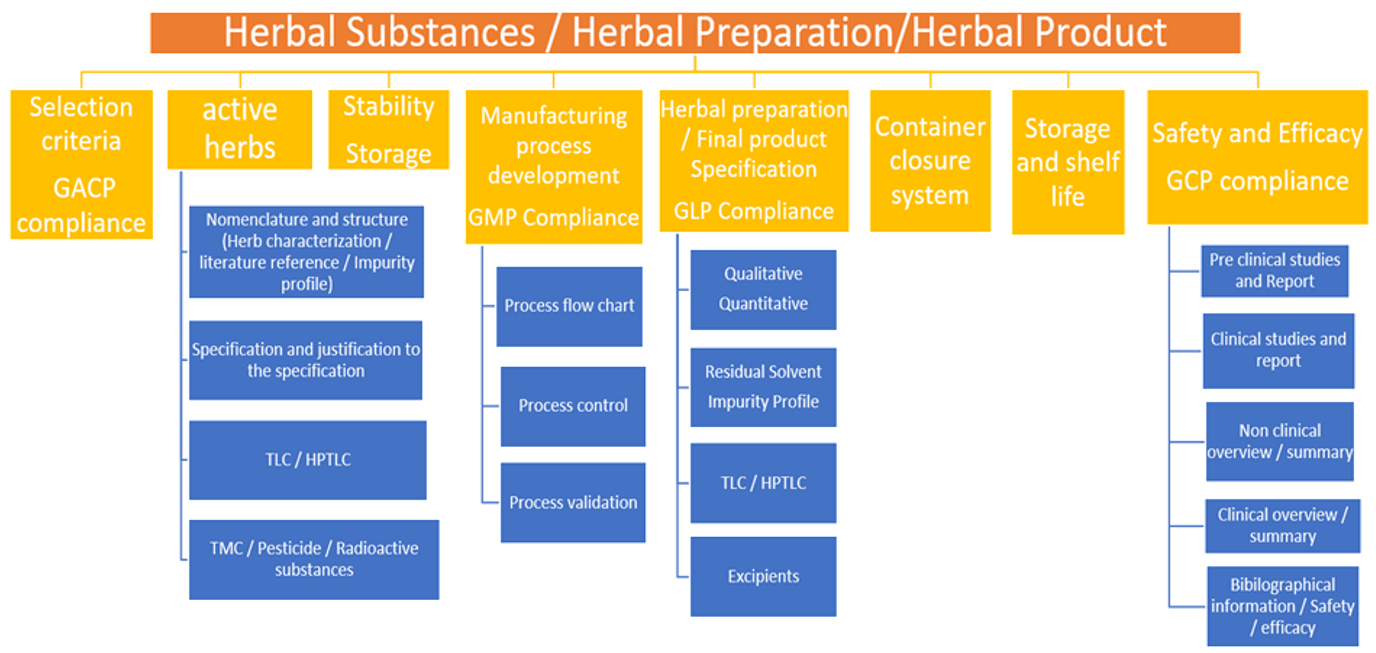
Dietary Supplement Good Manufacturing Practices Preparing For Compliance Pdf
Sep 26 2020 dietary supplement good manufacturing practices preparing for compliance posted by john grishamlibrary text id f721b3ed online pdf ebook epub library dietary supplements manufacturing and distribution.
Dietary supplement good manufacturing practices preparing for compliance pdf. For example distributors should undertake audits on the manufacturers to ensure compliance with good manufacturing practice. Bill mead s primer dietary supplement good manufacturing practices preparing for compliance should be required reading for every quality control department in the supplement industry. All businesses along the food supplement supply chain should ensure that the relevant sections are complied with.
Infant formula 21 cfr part 106. Preparing for compliance about the author dietary supplement good manufacturing practices is a one stop road map to the final dietary supplement gmp regulations issued by the fda food and drug administration covering the manufacture packaging labeling and holding of dietary supplement products. Sep 15 2020 dietary supplement good manufacturing practices preparing for compliance posted by richard scarrylibrary text id f721b3ed online pdf ebook epub library 21 cfr part 111 current good manufacturing practice in manufacturing packaging labeling or holding operations for dietary supplements cfr prev next subpart a general provisions 1111 1115 subpart b.
Nsf ansi 455 2 good manufacturing practices for dietary supplements audit template the audit template is a tool used by auditors to assist in their on site verification activity for compliance to the standard. The book dissects and digests the fda s lengthy regulations and preamble making the minutiae of a very complicated rulemaking understandable so the. Small entity compliance guide december 2010 content current as of.
Preparing for compliance william j. Mead dietary supplement gmp is a one stop how to road map to the final dietary supplement gmp regulations recently issued by the fda covering the manufacture packaging and holding of dietary supplement products. Dietary supplement good manufacturing practices.
Dietary supplements 21 cfr part 111 for additional information see current good manufacturing practices cgmps for dietary supplements. Current good manufacturing practice in manufacturing packaging labeling or holding operations for dietary supplements.