Ftc Guidelines For Dietary Supplement Advertising Claims

Identifying claims and interpreting ad meaning 1.
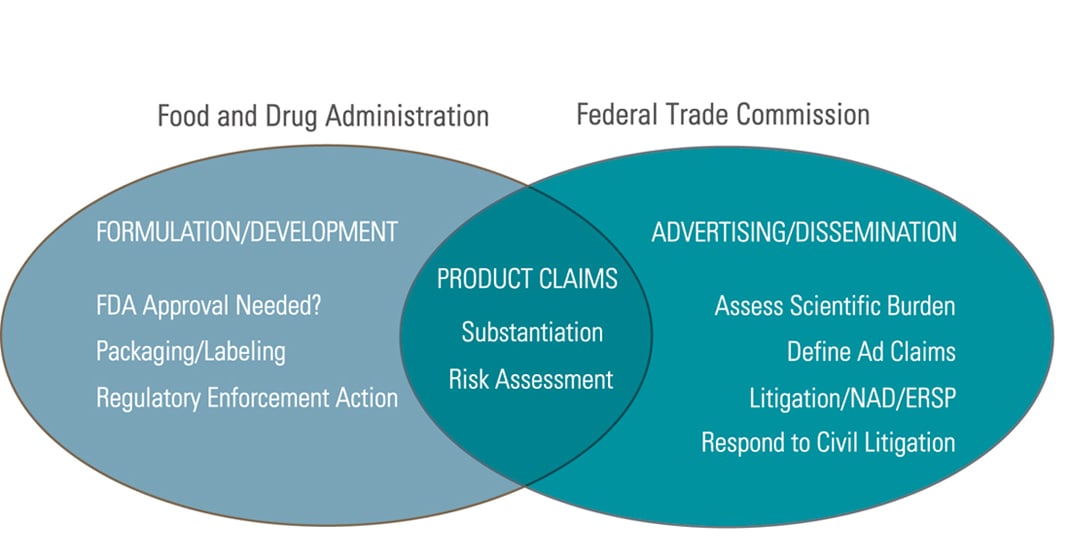
Ftc guidelines for dietary supplement advertising claims. When to disclose qualifying information 3. Application of ftc law to dietary supplement advertising a. Marketers of dietary supplements should be familiar with the requirements under both dshea and the ftc act that labeling and advertising claims be truthful not misleading and substantiated.
Identifying express and implied claims 2. The ftc has primary responsibility for claims in advertising and has primary jurisdiction over the advertising of foods nonprescription drugs cosmetics along with most other goods and services. It also has jurisdiction over the advertising of dietary supplements which the 1994 dietary su.
The federal trade commission ftc has primary jurisdiction over the advertising of foods nonprescription drugs cosmetics devices and services that are marketed in interstate commerce. An advertising guide for industry the dietary supplement guide. Given the dramatic increase in the volume and variety of dietary supplement advertising in recent years ftc staff is issuing this guide to clarify how.