Fda Definition Of Dietary Supplement
The nutrition labeling and education act of 1990 added herbs or similar nutritional substances to the fda definition of.
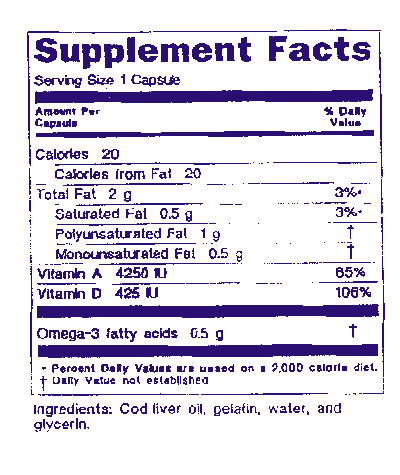
Fda definition of dietary supplement. How to use dietary supplement in a sentence. Fda regulates dietary supplements under a different set of regulations than those covering conventional foods and. The law defines dietary supplements in part as products taken by mouth that contain a dietary ingredient dietary ingredients include vitamins minerals amino acids and herbs or botanicals as.
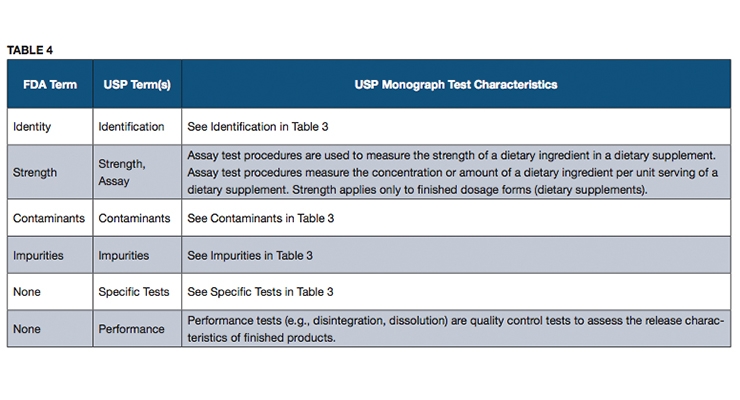
Fda regulates both finished dietary supplement products and dietary ingredients. The fda used to consider dietary supplements to be composed only of essential nutrients such as vitamins minerals and proteins. In addition the fda dietary supplement ingredient advisory list is intended to quickly alert the public when the fda identifies ingredients that do not appear to be lawfully included in products.
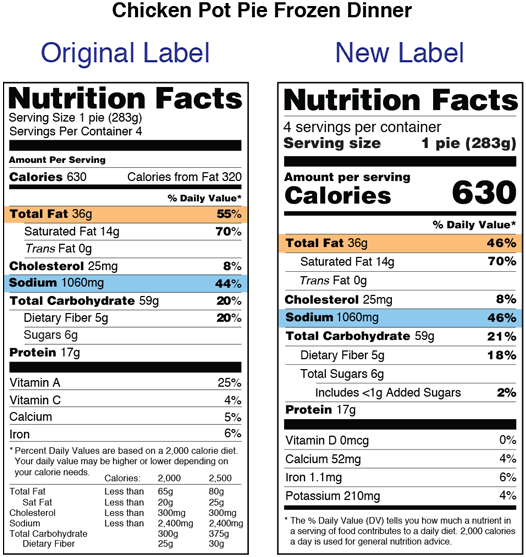
Dietary supplements are defined in part as products other than tobacco intended to supplement the diet that bear or contain one or more of the following dietary ingredients. Fda announced the nutrition and supplement facts label final rule which included a definition of dietary fiber and identified seven isolated or synthetic non digestible. A food or dietary supplement for which a claim subject to sections 343 r 1 b and 343 r 3 of this title or sections 343 r 1 b and 343 r 5 d of this title is made in accordance with the requirements of section 343 r of this title is not a drug solely because the label or the labeling contains such a claim.