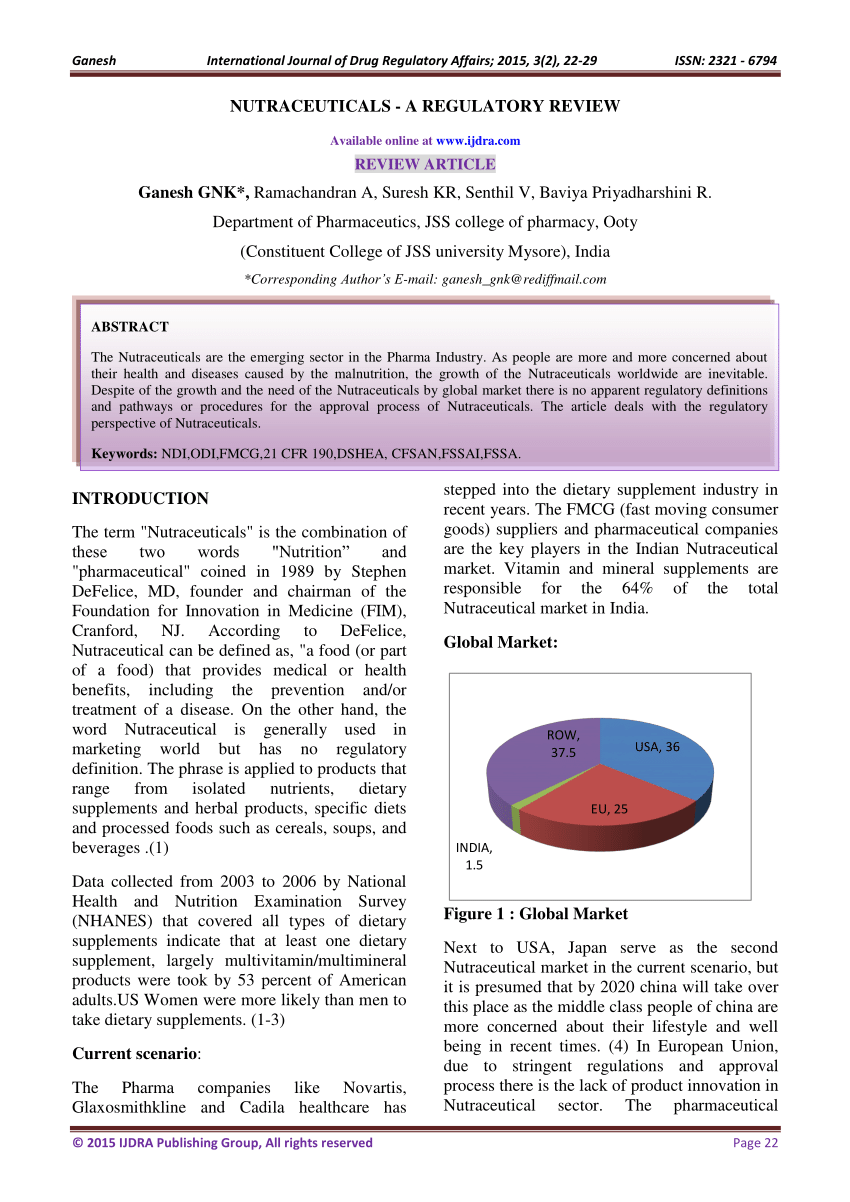
Dietary Supplement Safety Evaluation

Ideally a critical safety evaluation for each dietary supplement ingredient could eventually be completed but to best leverage available resources it is necessary to determine which supplement ingredients warrant attention first.
Dietary supplement safety evaluation. The screening flagging and priority setting steps outlined in the previous chapters will result in the identification of a group of dietary supplement ingredients that are of highest priority for further evaluation. No premarket assessment of the safety and composition of food supplements is however required. The first two steps in the process screening flagging and priority setting are designed to set priorities for.
Dietary supplements are marketed in forms such as tablets capsules softgels gelcaps powders. The committee on the framework for evaluating the safety of dietary supplements was asked to develop a framework for use by the food and drug administration fda to evaluate the safety of dietary supplement ingredients see appendix b for scope of work. Plant food supplements botanical preparations need to be safe according to the general food law and the herbal preparations decree under the dutch commodities act.
It was to include from a science based perspective a system for prioritizing review of dietary supplement ingredients that could be. Based on this experience and on comments received by industry. The second phase of the food and drug administration s fda charge to the committee on the framework for evaluating the safety of dietary supplements is to after proposing a framework for the safety evaluation of dietary supplement ingredients develop at least six monographs as prototypes for the system outlined in the framework.
This further evaluation requires the collection of additional information and critical analysis of the available safety data by the food and drug administration fda step 3 a. The senator s statement about the institute of medicine of the national academies refers to a recent publication suggesting a new framework for evaluating the safety of dietary supplement. Supplement safety supplements are not regulated by the fda in the same way that food and drugs are.