Dietary Supplement Manufacturing Sops

Manufacturing practices gmp requirements for manufacturers of dietary supplements nsf ansi 455 2 cosmetics and personal care products nsf ansi 455 3 and over the counter drugs nsf ansi 455 4.
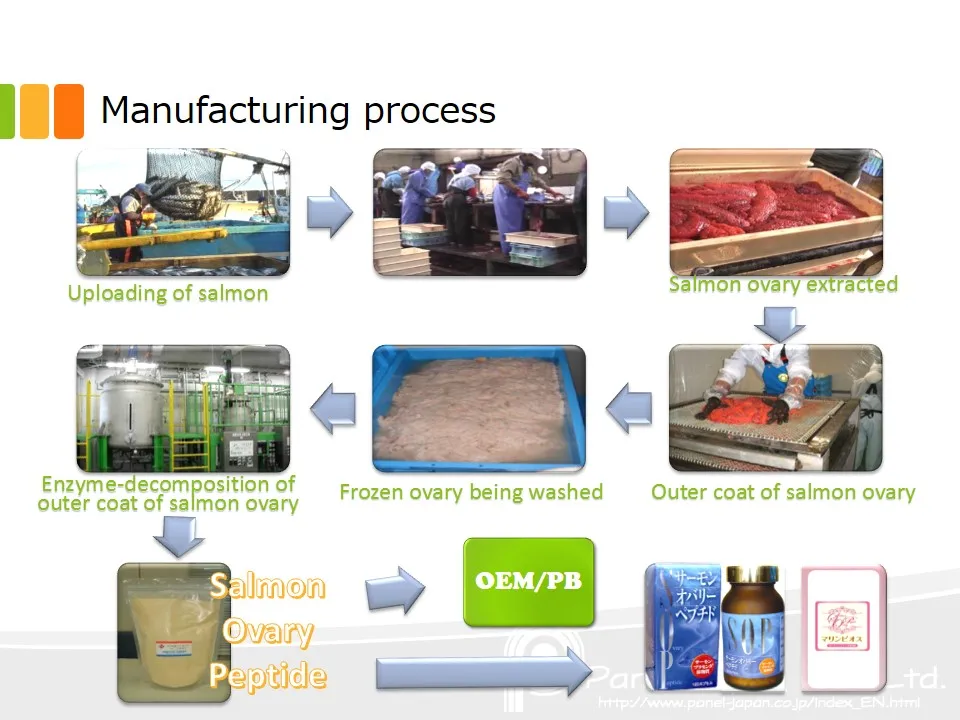
Dietary supplement manufacturing sops. But that doesn t mean complying with all the necessary part 111 provisions is easy or straightforward. It can be used for manual and automated tasks and also functions as a guide for safe work practices. Finding a manufacturer in this series we are briefly going over each of the steps we are assisting a new client with creating and launching their new dietary supplement product for their new dietary supplement company.
Services for over 25 years cosmax nbt has been a leading contract manufacturer for health supplements. Vincent tricarico is currently the vice president of contract manufacturing at nutrascience labs. The fda mandates that companies that manufacture and or distribute dietary supplements herbal products like hemp cbd kratom pharmaceuticals implement and follow a full set of sops as part of a quality system.
Perhaps to those involved in pharmaceutical manufacturing or clinical research the regulatory burden placed on holders and distributors of dietary supplements may seem light. Successful products for our customers our customer service will easily show why we are the choice company for supplement contract manufacturing. Dietary supplement sops starting a supplement company part 3.
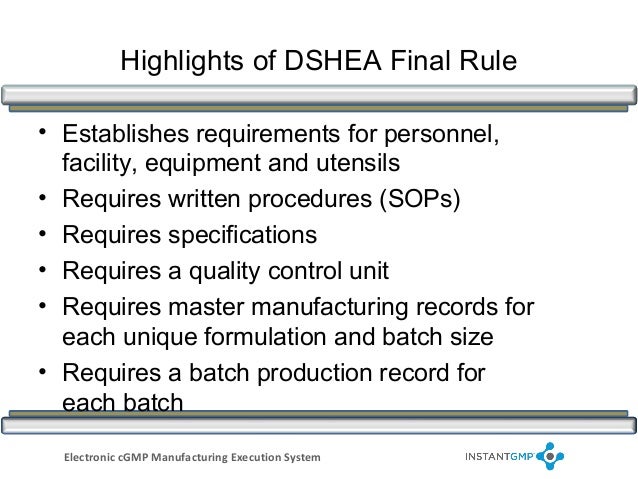
With nearly 20 years of direct to consumer and business to business experience mr. In addition an audit requirements guideline arg were published as companion documents to assist with the interpretation of each standard. The fda requires compliance with gmps in manufacturing packaging labeling and holding operations for dietary supplements and pharmaceuticals.
In fairness comparatively it is light. With a global footprint and the ability to create a wide range of product types. Compliance with manufacturing sops helps promote work consistency prevent loss of quality and.
A complete range of sops to comply with fda 21 cfr 111 and european food and dietary supplement gmp regulations. A manufacturing standard operating procedure sop is a set of documented instructions created to help workers perform routine manufacturing tasks. System long cycle times for qa review and corrections training and compliance to sops is lengthy documentation coordination and data tracking is laborious high cost of compliance instantgmp is the manufacturing system that has been built from the ground up to meet these challengesinstantgmp.