Dietary Supplement Labeling Cfr

It s a jungle out there or as some say comply or die.
Dietary supplement labeling cfr. Be sure to contact an experienced fda lawyer who understands the ins and outs of dietary supplement labeling. In the net quantity of contents statement because they tend to exaggerate the amount of the dietary supplement in the container. Keywords dietary supplements fda food package labels introduction package labels need to comply with labeling requirements set forth by the code of federal regulations cfr and with the need to protect the public from misleading health claims and from dangerous ingredients.
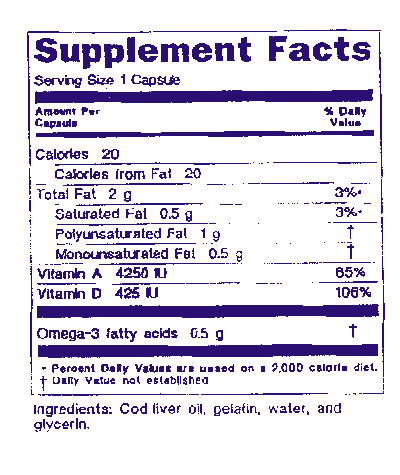
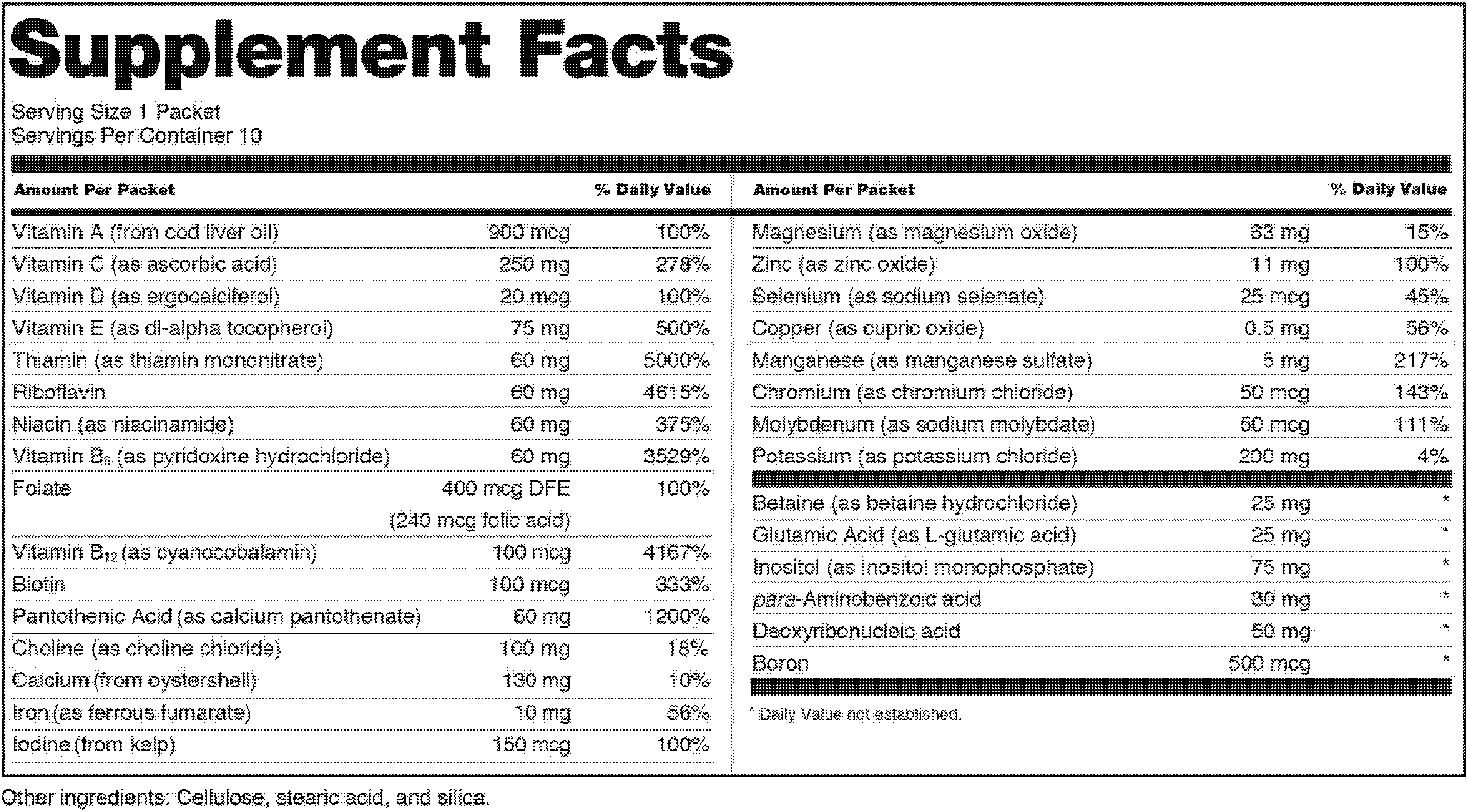
21 cfr 101. 21 cfr 101 36 b 1 i how does supplement facts differ from. Fda can take a variety of enforcement measures not limited to warning letters.
The label of a dietary supplement that is offered for sale shall bear nutrition labeling in accordance with this regulation unless an exemption is provided for the product in paragraph h of this section. 1 nutrition labeling information should be displayed at the point of purchase by an appropriate means such as by a label affixed to the food or through labeling including shelf labels signs posters brochures notebooks or leaflets that are readily. Regarding the need to protect the public.
7 21 cfr 101 3 a 8 21 cfr 101 105 a 9 21 cfr 101 36. A nutrition labeling for raw fruits vegetables and fish listed in 101 44 should be presented to the public in the following manner. 21cfr101 36 title 21 food and drugs.