Dietary Supplement Gmp Audit Checklist

Nsf ansi 455 2 good manufacturing practices for dietary supplements audit requirements guideline arg the document was developed to assist auditors and manufacturers to understand and interpret the requirements of the nsf ansi 455 2 gmp standard.
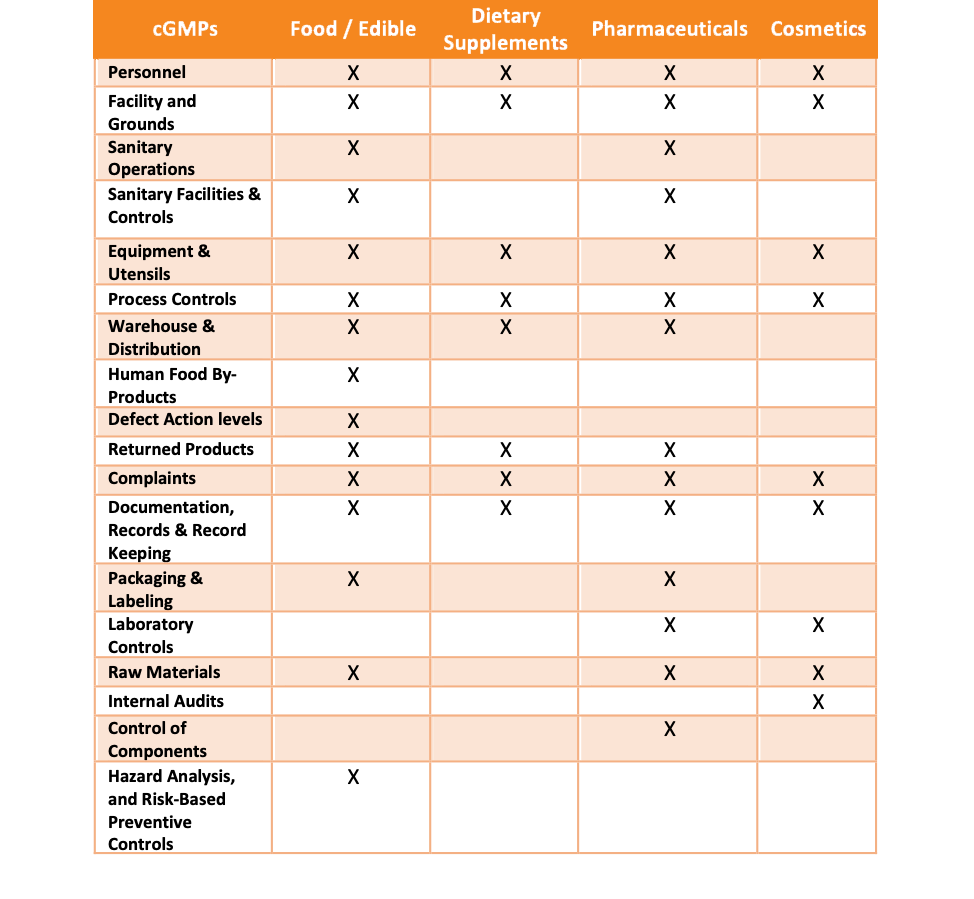
Dietary supplement gmp audit checklist. Gmp food manufacturing audit checklist. Dietary supplement audit checklist in accordance with cfr title 21 part 111 nsf ansi 173. This guideline should not be.
Section subpart b personnel. Current good manufacturing practice in manufacturing packing labeling or holding operations for dietary supplements. Manufacturers not being compliant with gmps.
Plus our easy to use detailed step by step implementation plan for food or dietary supplement gmp which can also be used for internal audits self inspection and monitoring. Audit evaluation yes no n a. The information in the guideline reflects the most.
The dietary supplement component supplier qualification is a voluntary guideline for information purposes only and intended to assist users with compliance with the current good manufacturing practice for dietary supplements 21 c f r. Since then the number of fda gmp compliance audits has increased every year. Identify problem areas and assign immediate corrective actions by performing your food manufacturing audits with this template using the iauditor mobile app.
Through its audits of supplement manufacturers fda has pointed out a kind of adulteration within our industry. Contract manufacturing audit checklist for supplement brand owners tags. The final rule on dietary supplement gmps good manufacturing practices was issued in 2007.
Federal register for the final rule june 25 2007. 111 10 a procedures have been established that define work requirements for personnel to prevent microbial contamination from illness and or hygienic practices. Business resources supplement brands looking to select their contract manufacturer need to see a slew of paperwork the production facility and the testing labs before they can assess whether a partner is the right fit.