Dietary Supplement Cfr Fda

I dietary ingredients for which fda has not established rdi s or drv s and that are not subject to regulation under paragraph b 2 of this section hereinafter referred to as other dietary ingredients shall be declared by their common or usual name when they are present in a dietary supplement in a column that is under the column of.
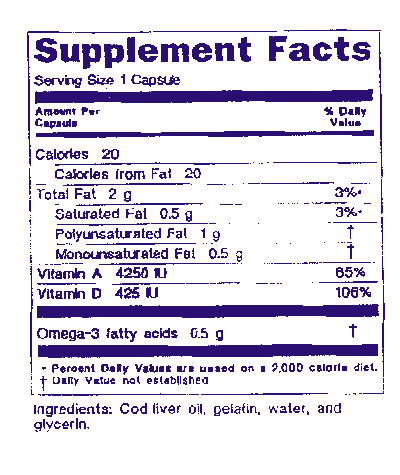
Dietary supplement cfr fda. 111 470 what requirements apply to distributing dietary supplements. Office of dietary supplement programs hfs 810 food and drug administration 5001 campus dr college park md 20740. The dv must be declared for all dietary ingredients for which fda has established daily values except that 1 the percent for protein may be omitted and 2 on the labels of dietary supplements.
Part 111 current good manufacturing practice in manufacturing packaging labeling or holding operations for dietary supplements subpart m holding and distributing sec. Dietary supplements 21 cfr part 111 for additional information see current good manufacturing practices cgmps for dietary supplements. 111 165 what requirements apply to a product received for packaging or labeling as a dietary supplement and for distribution rather than for return to the supplier.
Title 21 food and drugs. Dietary supplements 21 cfr 111 part 111 current good manufacturing practice in manufacturing packaging labeling or holding operations for dietary supplements subpart a general provisions. Part 111 current good manufacturing practice in manufacturing packaging labeling or holding operations for dietary supplements.
You must take measures to exclude from any operations any person who might be a source of microbial contamination due to a health condition where such contamination may occur of any material including components dietary supplements and contact surfaces used in the manufacture packaging labeling or holding of a dietary supplement. Code of federal regulations title 21 volume 2. Chapter i food and drug administration department of health and human services.
111 170 what requirements apply to rejected components packaging and labels and to rejected products that are received for packaging or labeling as a dietary supplement. To contact the office of dietary supplement programs email. A preventing microbial contamination.