Dietary Supplement Advertising Law

Whether labeling and advertising claims for multi ingredient dietary supplements may be based on the testing of individual key ingredients rather than the actual product has caused a good deal of confusion.
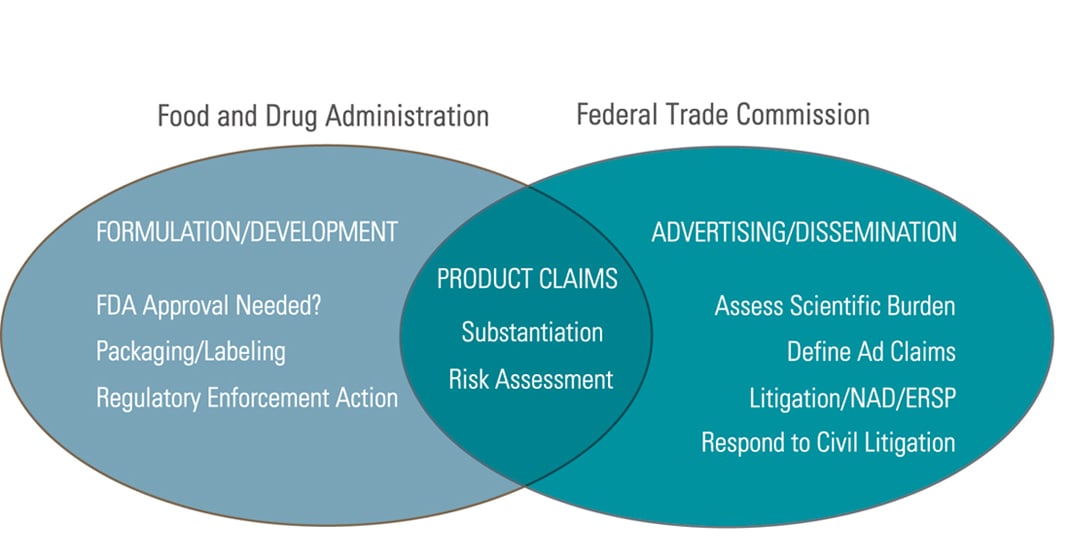
Dietary supplement advertising law. Given the dramatic increase in the volume and variety of dietary supplement advertising in recent years ftc staff is issuing this guide to clarify how long standing ftc policies and enforcement practices relate to dietary supplement advertising. This confusion stems from the dearth of case law and the open endedness of federal trade c. Under the dietary supplement health and education act of 1994 dshea.
Dietary supplements are regulated under the dshea dietary supplements health education act of 1994 which is incorporated into the fdca and cosmetics under the dshea as well as the fair packaging and labeling act fpla. As the market. Application of ftc law to dietary supplement advertising the ftc s truth in advertising law can be boiled down to two common sense propositions.
Since the dietary supplement health and education act dshea was passed 26 years ago the dietary supplement industry has been reshaped by a complex global supply chain the internet and newly discovered ingredients with unknown safety according to an ama council on. While millions of patients use dietary supplements the current regulatory structure does not properly protect the public. Dietary supplement advertising including ads broadcast on radio and television falls under the jurisdiction of the federal trade commission.
And 2 before disseminating an ad advertisers must have adequate substantiation for all objective product claims. The fdca prohibits the marketing of adulterated or misbranded dietary supplements and cosmetics in interstate commerce. The supplement industry in the united states has surpassed 37 billion dollars in annual sales and is growing at a rate of 7 10 annually.
As the american public becomes more health conscious and informed more consumers are taking their health into their own hands and using dietary supplements to improve their lives. Manufacturers and distributors of dietary supplements and dietary ingredients are prohibited from marketing products that are.