The Dietary Supplement And Education Act Of 1994 Primarily Pertains To

103 41 7 summary since passage of the 1990 nutrition labeling and education act nlea p l.
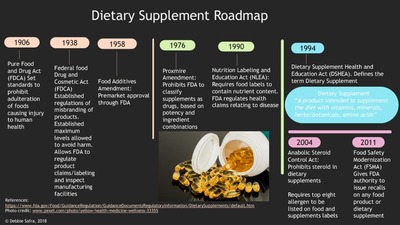
The dietary supplement and education act of 1994 primarily pertains to. Under the act supplements are effectively regulated by the fda for good manufacturing practices under 21 cfr part 111. In the united states dietary supplements are defined by the 1994 dietary supplement health and education act as products that are not used exclusively as food but are intended to be consumed in addition to an individual s diet. The dietary supplement health and education act of 1994 dshea is a 1994 statute of united states federal legislation which defines and regulates dietary supplements.
101 585 there h d s been considerable controversy about the regulation or dietary supplements under that act. Under the act supplements are effectively regulated by the fda for good manufacturing practices under 21 cfr part 111. The dietary supplement health and education act of 1994 dshea is a 1994 statute of united states federal legislation which defines and regulates dietary supplements.
This summary is from wikipedia. This act may be cited as the dietary supplement health and education act of 1994. Whenever in this act an amendment or repeal is expressed in terms of an amendment to or repeal of a section or other provision the reference shall be considered to be made to a section or other provision of the federal food drug and cosmetic act.
Its labeling requirements include ingredients and nutritional content.