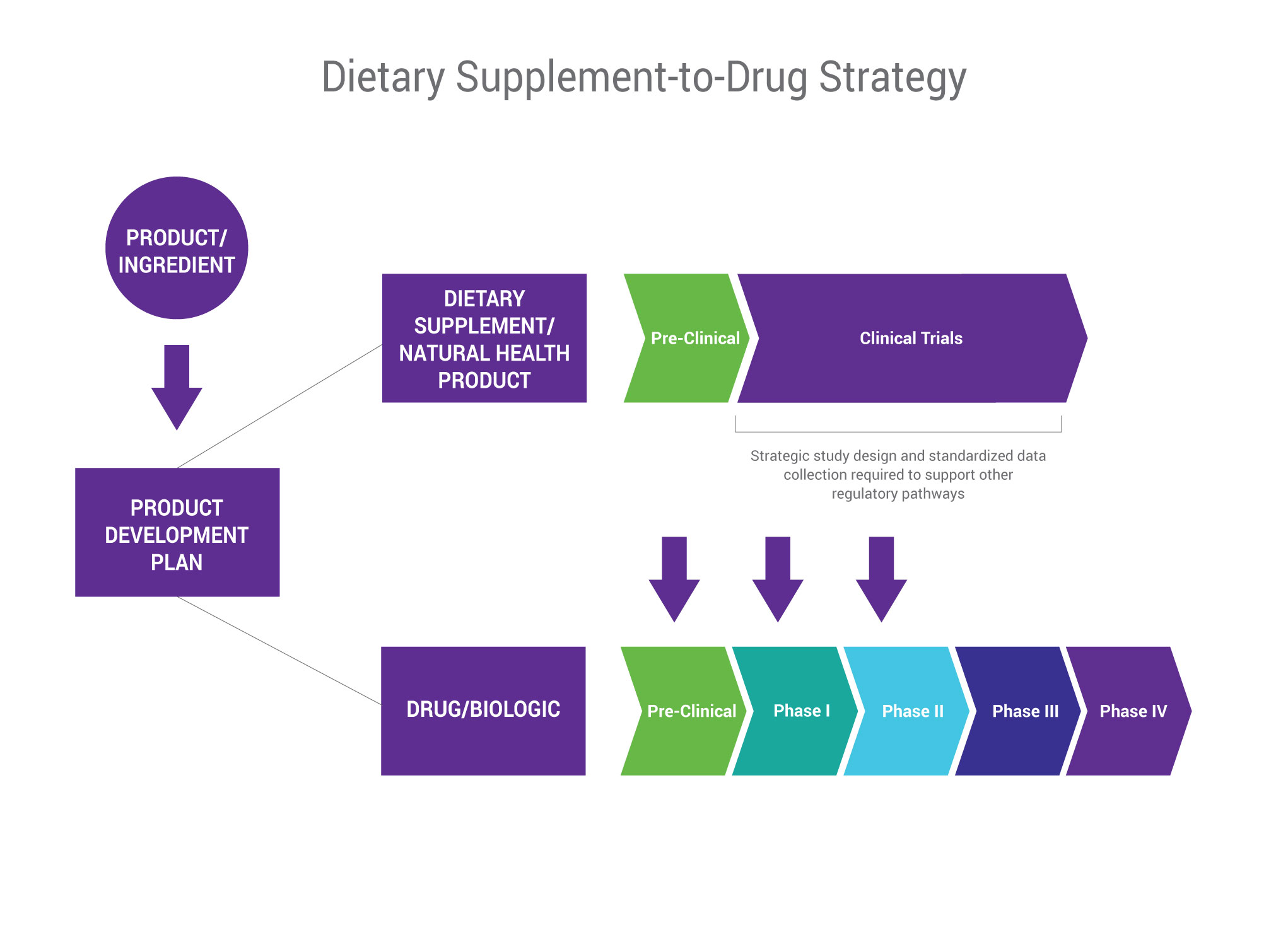
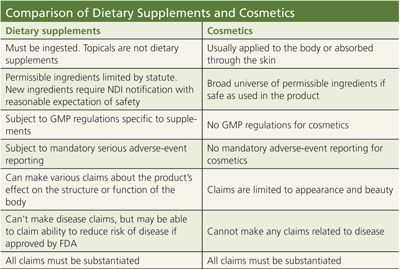
Fda Dietary Supplement Claim Substantiation

The quality of the evidence.
Fda dietary supplement claim substantiation. But it needs to keep claims on the right side of the line and stay out of the crosshairs of the government. Substantiation for dietary supplement claims made under section 403 r 6 of the federal food drug and cosmetic act january 2009. Service s related to this article.
The meaning of the claim s being made. The totality of the. 1 the 1990.
A low calorie claim may not be made on dietary supplements except when an equivalent amount of a dietary supplement that the labeled dietary supplement resembles and for which it substitutes e. Substantiation for dietary supplement claims although fda eschews a particular formula for the number and type of studies required to substantiate a claim it suggests that advertisers consider. There are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement.
The federal trade commission is the federal agency that regulates dietary supplement advertising claims. Structure function claims for dietary supplements may describe how a nutrient or dietary ingredient is intended to affect the normal structure or function of the human body or the way an ingredient acts to maintain a bodily structure or function. 1 888 safefood 1 888 723 3366 guidance for industry.
For more information about the difference between structure function claims and disease claims see 21 cfr 101 93 entitled certain types of statements for dietary supplements and fda s. A supplement brand has developed a great product and wants to advertise the benefits of that product to the world. This guidance represents fda s current thinking on the substantiation for dietary supplement claims made under section 403 r 6 of the act.
Contact the office of dietary supplement programs by email at sfcn fda hhs gov to reach fda s food and cosmetics information center call. The relationship of the evidence to the claim.