Dietary Supplements Fda Clearance

A dietary supplement is a product taken by mouth that contains a dietary ingredient intended to supplement the diet the fda adds that the dietary ingredients inside supplements can include vitamins minerals herbs or other botanicals amino acids and substances such as enzymes organ tissues glandulars and.
Dietary supplements fda clearance. Notify fda if the use of a dietary supplement caused you or a family member to have a serious reaction or illness even if you are not certain that the product was the cause or you did not visit a. Other blogs fda was abbreviated as the food and drug administration. Regulatory fda compliance.
To contact the office of dietary supplement programs email. The company could get fda clearance if it can compare its product to another that s already on the market and demonstrate that it is. Dietary supplement brands the recall may apply to dietary supplements marketed by about 850 u s.
Duties of fda dietary supplement health and education act by kelly shelton 12 27 2019. Office of dietary supplement programs hfs 810 food and drug administration 5001 campus dr college park md 20740. The duty fda is to evaluate and approves many different things such as drug food items and some medical devices.
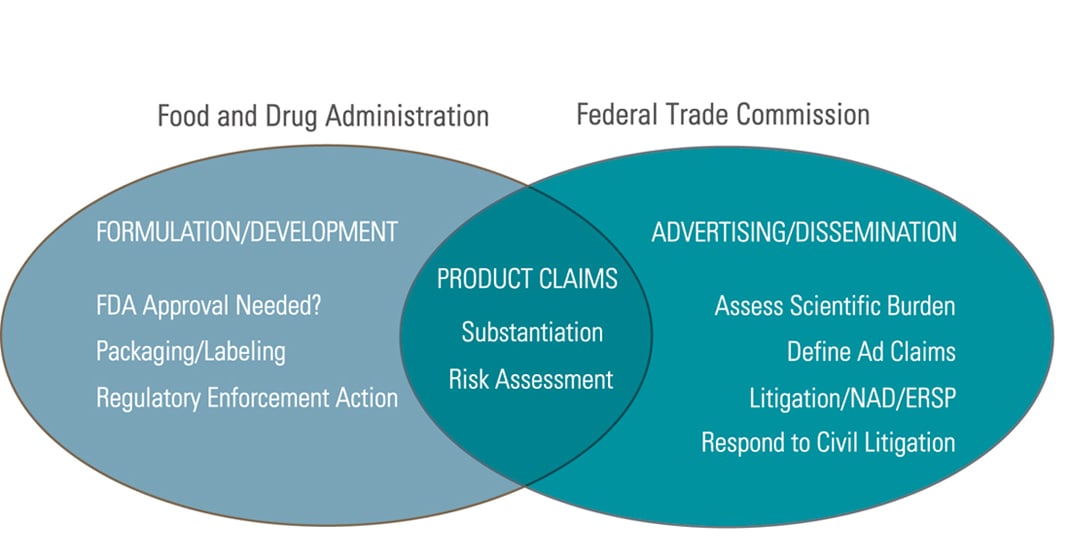
Fda is disappointed with continuing violations of basic manufacturing requirements applicable to the dietary supplement industry. If you experience such an adverse effect contact or see your health care professional immediately. Understanding regulatory and fda compliance with dietary supplements advertising first means understanding that the food drug administration the fda has primary responsibility for claims related to dietary supplements and weight loss products on product labeling including packaging inserts and other promotional materials distributed at the point of sale.
Recently a new york based dietary supplement manufacturer and its subsidiaries issued a nationwide recall of all lots of dietary supplement products manufactured and sold between january 2013 november 2019 because the manufacturer previously supplied dietary supplements to a large number of u s. Guidance and regulatory information on food and dietary supplements. In fiscal year 2019 fy19 dietary supplement manufacturing facilities issued an inspection report or so called form 483 for violations of cgmps current good manufacturing practices were most commonly cited for failing to establish specifications for.