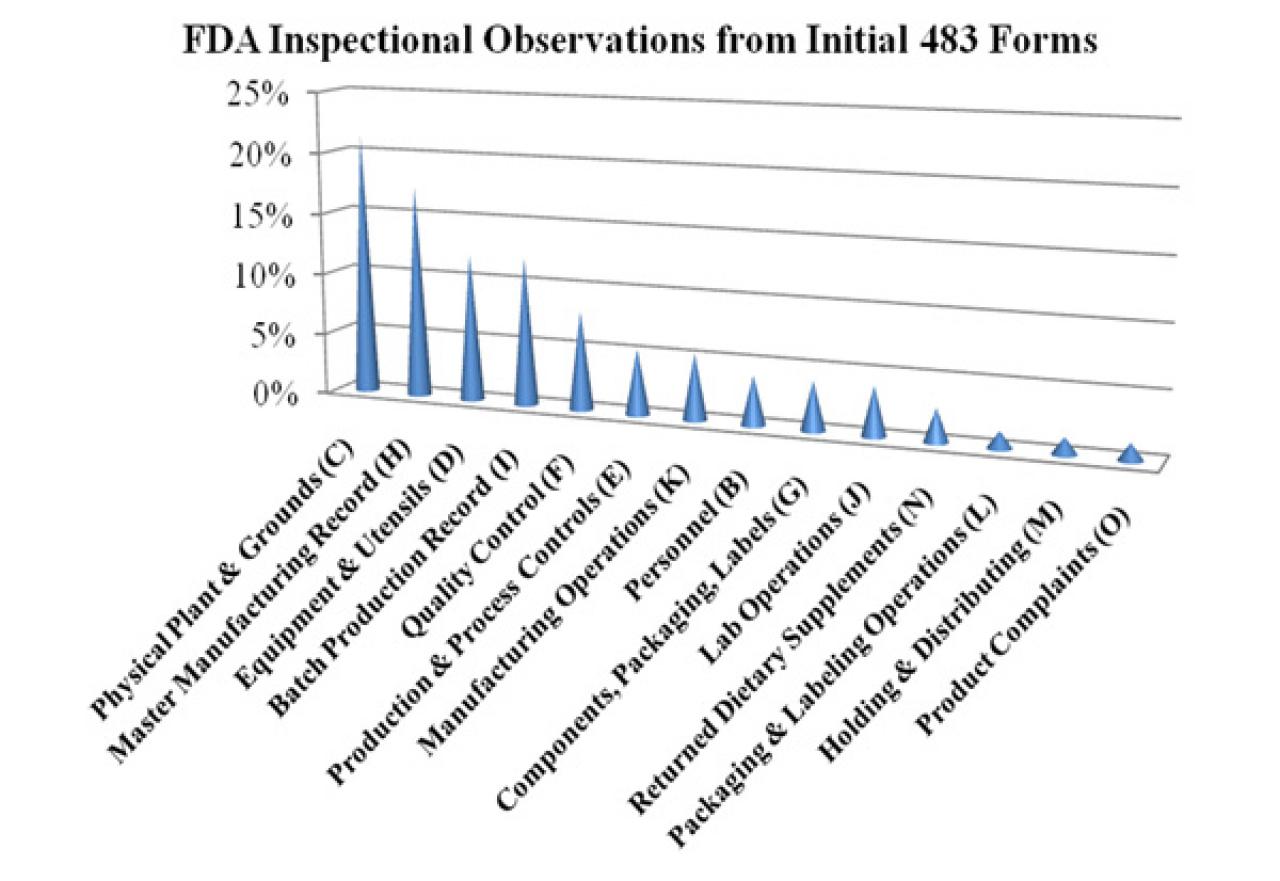
Dietary Supplement Master Manufacturing Record

Procedures for sampling and a cross reference to procedures for tests or examinations.
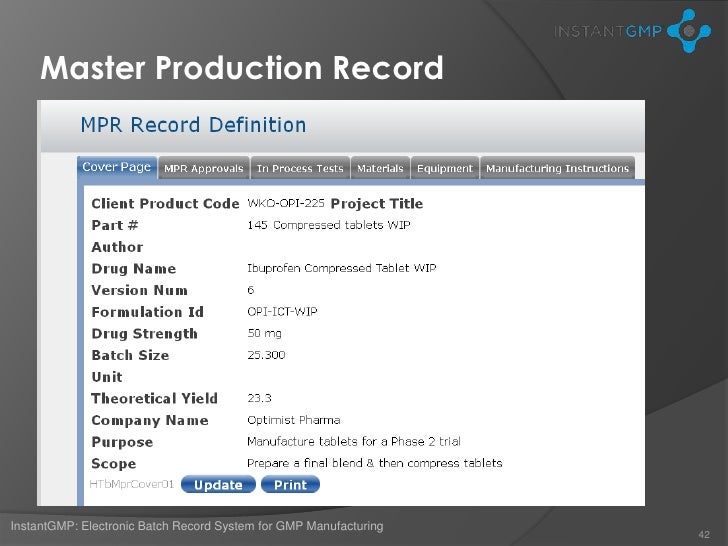
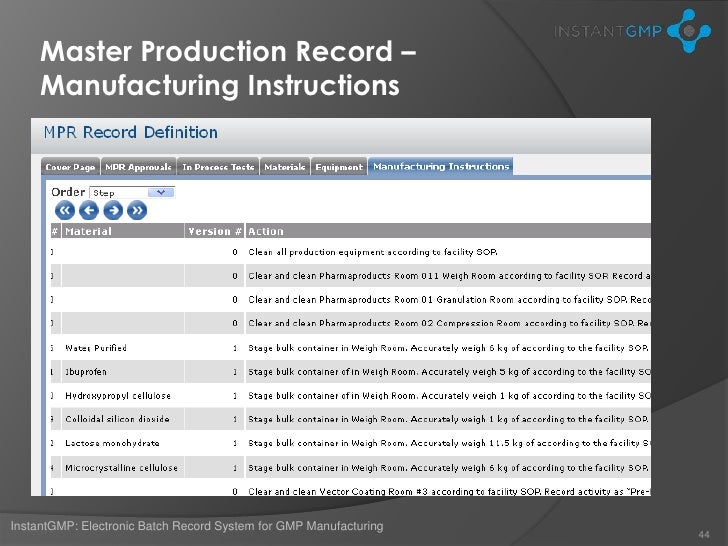
Dietary supplement master manufacturing record. The batch production record must accurately follow the appropriate master manufacturing record. Specifications for each point step or stage in the manufacturing process where control is necessary to ensure the quality of the dietary supplement and that the dietary supplement is packaged and labeled as specified in the master manufacturing record. Hazard analysis critical control points haccp.
1 identify specifications for the points steps or stages in the manufacturing process where control is necessary to ensure the quality of the dietary supplement and that the dietary supplement is packaged and labeled as specified in the master manufacturing record. Mmrs identify steps and stages in the manufacturing process for each supplement to ensure consistency in the components quality labeling and packaging of the. The master manufacturing records ensure your manufacturing process is performed consistently and uniformly from batch to batch.
It is required by 111 205 to establish written master manufacturing records mmrs a vital part of the production and process control system. Dietary supplement manufacturing facilities are required to create these documents and keep them on file but what exactly is the. A the name of the dietary supplement to be manufactured and the strength concentration weight or measure of each dietary ingredient for each batch size.
You must also have a batch production record bpr every time a dietary supplement batch is made. B a complete list of components to be used. C an accurate statement of the weight or measure of each component to be used.
The presentation provides an overview of these requirements. Master batch records also known as master production records and master manufacturing records are version controlled templates for your manufacturing process. It also ensures that processed food or dietary supplements are packaged and labeled as specified in the master manufacturing record.
D the identity and weight or measure of each dietary ingredient. Registrar corp s food safety specialists can review your master manufacturing record for fda compliance. B the master manufacturing record must.