Dietary Supplement Labeling Guide Chapter Iv

What is the name of the food statement called and where must it be placed.
Dietary supplement labeling guide chapter iv. Should the statement of identity stand out. Name of food contains nonbinding recommendations 1. What is the name of the food statement called and where must it be placed.
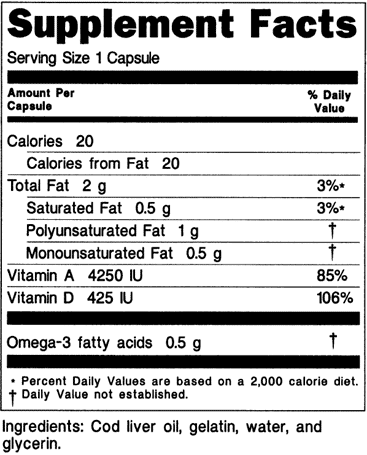
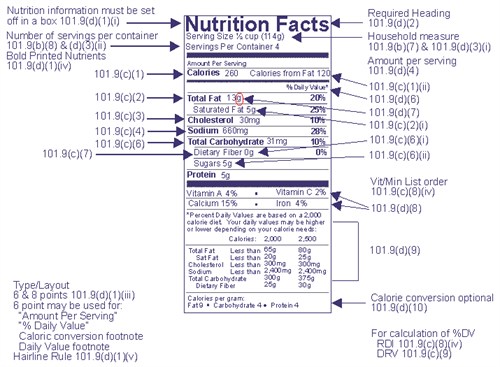
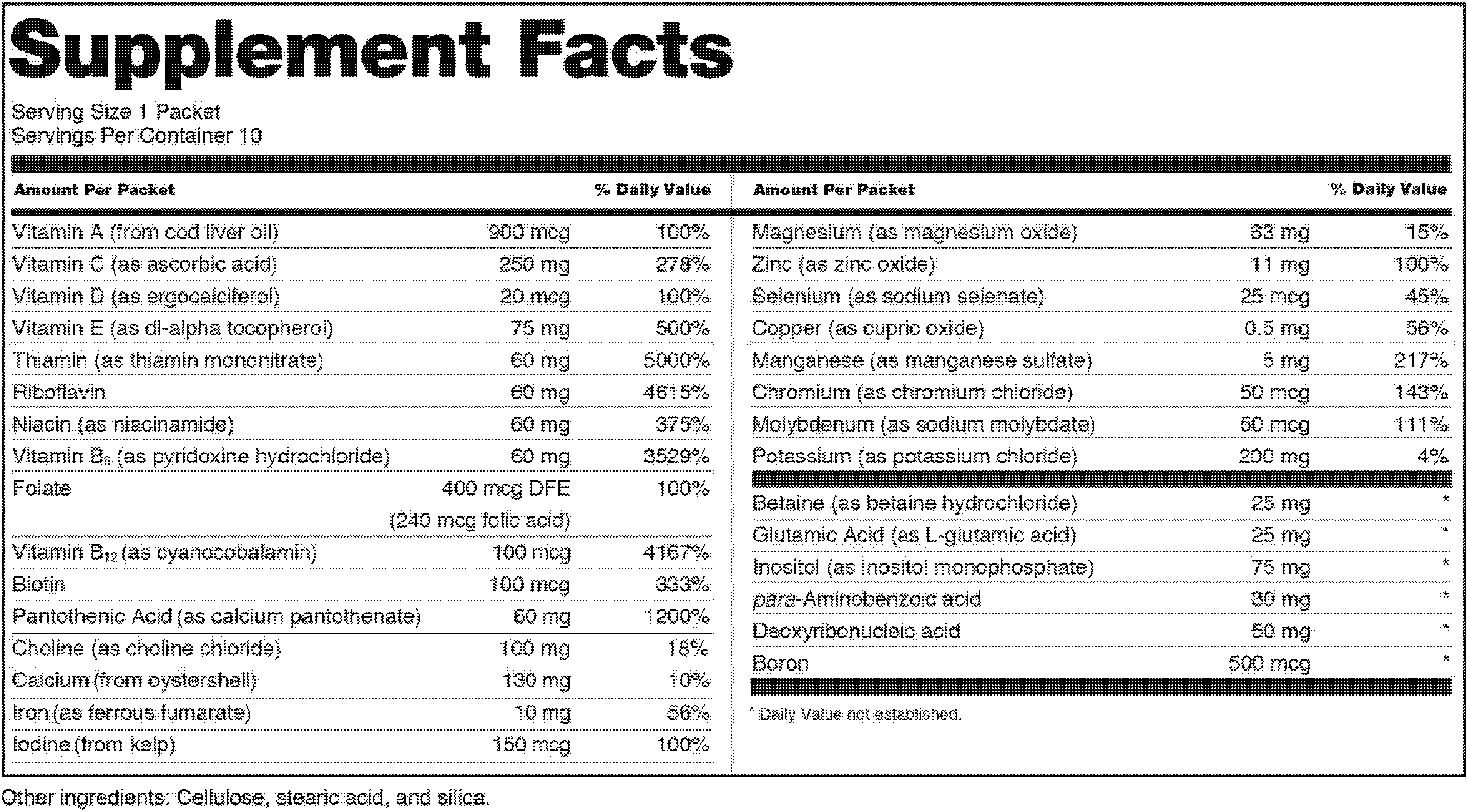
1 the statement of identity name of the dietary supplement 2 the net quantity of contents statement amount of the dietary supplement 3 the nutrition labeling 4 the ingredient list and 5 the name and place of business of the manufacturer packer or distributor. A dietary supplement labeling guide chapter iv. The dietary supplement health and education act of 1994 the dshea amended the act in part by defining dietary supplements adding specific labeling requirements for dietary supplements and.
1 nutrition labeling information should be displayed at the point of purchase by an appropriate means such as by a label affixed to the food or through labeling including shelf labels signs posters brochures notebooks or leaflets that are readily. Should the statement of identity stand out. The gov means it s official.
Name of food contains nonbinding recommendations 1. What name should be used as the statement. Cfsan office of nutrition labeling and dietary supplements april 2008 guidance for industry a food labeling guide chapter iv.