Dietary Supplement Label Review

Five general requirements for labels include.
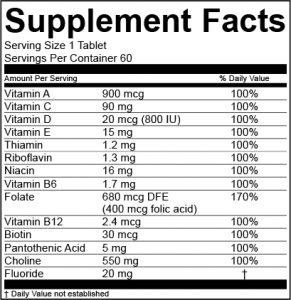
Dietary supplement label review. The supplement facts label includes most of the basic information about a supplement including the serving size number of servings per container ingredients in the product and amount of each. Verify that claims made on the label are appropriate structure. Our dietary supplement label review solution is fast simple and cost competitive.
Dietary supplement label review checklist packaging requirements. We will assist with fda compliance regarding foods dietary supplements drugs or medical devices. To maintain the product s status as a dietary supplement the label and labeling must be consistent with the provisions in the dietary supplement health and education act dshea of 1994 labeling.
We cover all of the following in our dietary supplement label review. 100 accuracy and fda compliance experience. This is the first of five review articles investigating dietary supplements ds.
Includes herbs that now exceed over 50 000 in the office of dietary supplement s dietary supplement label database four review articles follow summarizing published medical case reports of ds related to liver toxicity kidney toxicity heart toxicity and cancer. 1 the statement of identity name of the dietary supplement 2 the net quantity of contents statement amount of the dietary supplement 3 the nutrition labeling supplement facts panel 4 the ingredient list and 5 the. 1 the statement of identity name of the dietary supplement 2 the net quantity of contents statement amount of the dietary supplement 3 the nutrition labeling 4 the ingredient list and 5 the name and place of business of the manufacturer packer or distributor.
We provide a very quick turnaround on dietary supplement label reviews and will identify and correct any mistakes or enforcement risks before you print or ship your products. The dietary supplement health and education act of 1994 the dshea amended the act in part by defining dietary supplements adding specific labeling requirements for dietary supplements and. We can review or prepare labels and advise your company regarding products requiring fda ftc compliant labeling such as dietary supplements nutraceuticals or cosmetics.
As part of our label review services naturpro helps clients develop review and suggest improvements to dietary supplement labels to ensure compliance with fda regulatory requirements.